Research Article - Archives of Clinical and Experimental Surgery (2025)
BCG Lymphadenitis in Infants: Lessons Learned
Pooja Tiwari*, Maneesh Joleya, Vinod Raj, Geetesh Ratre, Nilakshi Devi Choudhury, Ram Mohan Shukla,, B. K. Lahot, Manoj Joshi, Shashi Shankar Sharma, Ashok Kumar Laddha and Ankur DeshwaliPooja Tiwari, Department of Pediatric Surgery, MGM Medical college, Indore, India, Email: drtiwaripooja@gmail.com
Received: 19-Jun-2025, Manuscript No. EJMACES-25-166969; Editor assigned: 24-Jun-2025, Pre QC No. EJMACES-25-166969 (PQ); Reviewed: 08-Jul-2025, QC No. EJMACES-25-166969; Revised: 15-Jul-2025, Manuscript No. EJMACES-25-166969 (R); Published: 14-Aug-2025
Abstract
Bacillus Calmette-Guerin (BCG) vaccination is a cornerstone of tuberculosis prevention. BCG lymphadenitis, either suppurative or non-suppurative, remains the most frequent adverse effect following vaccination.
Aims: Despite widespread neonatal BCG immunization in our nation, the incidence of BCG lymphadenitis remains poorly documented. Thus this study aims to analyze the clinical presentation, management approaches and outcomes of BCG lymphadenitis in infants, while establishing evidence-based treatment protocols.
Materials and methods: It is a retrospective observational study which included 54 infants with BCG lymphadenitis who visited the paediatric surgical outpatient department of our institution during 2 years’ period from March 2022 to March 2024. with standardized follow-up protocols and comprehensive diagnostic testing.
Results: Among 54 cases (37 male, 17 female; male: female ratio 2.18:1; 95% CI: 1.42-3.35), mean presentation age was 5.4 months (SD ± 2.3). CBNAAT positivity was observed in 31 cases (57.4%; 95% CI: 44.2-70.6%), with histopathological confirmation in 22 cases (40.7%; 95% CI: 27.6-53.8%). Aspiration of pus was done in 32 (59.3%) cases, excision in 18 cases (33.3%) which were refractory to multiple aspirations (more than twice), Incision and drainage and curettage was done in 4 cases (7.4%) which presented with ruptured abscess.
Conclusion: This study provides comprehensive data on BCG lymphadenitis presentations and treatment outcomes, contributing to standardized management protocols.
Keywords
BCG; Lymphadenitis; Suppurative; CBNAAT
Introduction
The BCG vaccination was first administered to humans in 1921 by French scientists Albert Calmette and Camille Guerin to combat pulmonary Tuberculosis (TB), a leading cause of mortality in the early 1900’s. Following its introduction in India in August 1948, BCG vaccination programs were established across most Indian states by 1949 and was incorporated into India’s Universal Immunisation Programme in 1985 [1].
Current protocols specify administration as a single intradermal injection at the deltoid insertion immediately after birth. The formation of a distinctive scar serves as evidence of successful vaccination.
Lymphadenitis represents the most common adverse event following BCG immunization. Recent meta analyses indicate a worldwide BCG lymphadenitis incidence of 0.1-17%, with significant regional variations. Environmental, genetic and technical factors contribute to this variability. A comprehensive understanding of these factors is crucial for prevention and management. Recent molecular studies have identified key immunological pathways involved in BCG lymphadenitis development.
Host factors
•Genetic polymorphisms in IL-12/IFN-γ pathway
• Age-related immune response variations prematurity and low birth weight at high risk.
• Nutritional status impact on immune function.
• Immunocompromised patients such as those suffering from SCID or IFN-g/IL-12 pathway defects, have increased rates of local complications as well as disseminated BCG infection.
Vaccine-related factors
• Strain-specific immunogenicity profiles.
• Dose-dependent immune responses.
• Viability of final vaccine product affected by storage conditions such as the cold chain.
• Residual virulence of the BCG strain.
Administration-related factors
• Subcutaneous rather than intradermal injection.
• Higher dose administered.
Non-suppurative lymphadenitis typically resolves spontaneously, while suppurative cases often require medical and surgical intervention.
Materials and Methods
It is a prospective study conducted for 2 years from March 2022 to March 2024, which included all infants with BCG lymphadenitis who visited the paediatric surgical Outpatient Department (OPD). Older children and children with lymphadenitis on contralateral side or with systemic or local signs of inflammation or cases of non-suppurative BCG lymphadenitis which resolved without treatment; were excluded from study. In order to track the progression of adenitis during the study period, the children were monitored and were followed up for a year. These children’s parents were negative for HIV infection [2,3].
Diagnostic criteria, based on current literature, included:
• Absence of systemic or local inflammatory signs.
• Isolated axillary or supraclavicular lymph node enlargement.
• Ipsilateral BCG vaccination site.
• Onset between 2 weeks and 6 months, most patients present within 2-4 months after BCG vaccination.
Results
Our analysis revealed 54 cases of BCG adenitis (37 males, 17 females), consistent with gender distributions reported in previous studies [4-7]. Age at presentation ranged from 2 months to 1 year (mean: 5.4 months), aligning with documented presentation patterns. The majority (72.2%, n=39) received vaccination at local Anganwadi centers (Table 1).
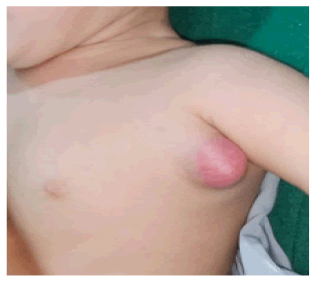
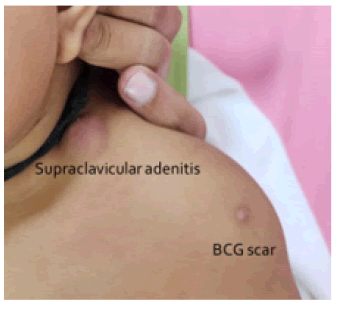
Clinical presentation included left axillary or supraclavicular nodes and BCG scar in all cases. 44 patients presented with left axillary and rest 10 with left supraclavicular lymphadenitis (Figures 1-3).
| Characteristics | Value | 95% CI |
|---|---|---|
| Male: Female Ratio | 2.18:1 | 1.42-3.35 |
| Mean age (months) | 5.4 | ± 2.3 |
| CBNAAT positive | 57.40% | 44.2%-70.6% |
| Histopathology positive | 40.70% | 27.6%-53.8% |
Table 1. Demographic and clinical characteristics.
Figure 1. Clinical image of left axillary suppurative BCG lymphadenitis.
Figure 2. Clinical image left supraclavicular suppurative BCG lymphadenitis.
Figure 3. Clinical image left non-suppurative axillary BCG lymphadenitis.
Cartridge-Based Nucleic Acid Amplification Test (CBNAAT) also known as Genexpert TB test of abscess material was performed according to standard protocols in all cases and showed positivity for Mycobacterial Tuberculosis(MTB) in 31 cases, with corresponding acid-fast bacilli on microscopy. All pus cultures remained sterile, consistent with previous reports. Histopathological examination revealed tubercular granuloma in 22 patients (20 CBNAAT positive, 2 negative) [8-10].
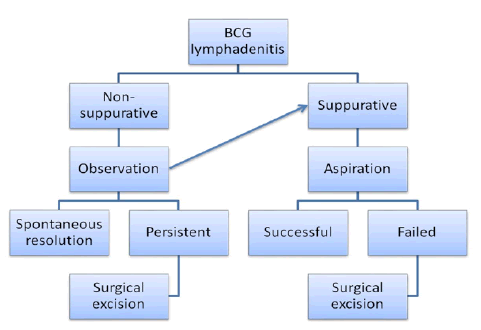
Of all 54 cases 4 were non-suppurative on presentations which were managed conservatively but required excision during study period due to non resolution and suppuration on follow up. Aspiration of pus was done in 32 (59.3%) cases, excision in 18 cases (33.3%) which were refractory to multiple aspirations (more than twice), incision and drainage and curettage were done in 4 cases (7.4%) which presented with ruptured abscess (Figure 4).
Figure 4. Flowchart for management.
Data analysis performed using SPSS version 26.0. Categorical variables were compared using χ2 test or Fisher’s exact test as appropriate. Continuous variables were analyzed using Student’s t-test or Mann-Whitney U test based on distribution normality. On clinical outcomes analysis, treatment efficacy analysis revealed:
• Aspiration success rate: 78.1% (25/32; 95% CI: 63.8%-92.4%).
• Surgical excision success rate: 94.4% (17/18; 95% CI: 83.9%-100%).
• Incision and drainage with Curettage success rate: 100% (4/4).
All CBNAAT and histopathology positive cases completed 6-month Anti-Tubercular Treatment (ATT) regimens. One patient with haemangioma at local BCG scar site and left axillary region with persistent left axillary BCG adenitis and ulceration was given empirical anti-tubercular treatment. One patient who had not complied with ATT had recurrence of suppurative adenitis and improved after completion of ATT. Though need for ATT in cases of BCG adenitis is controversial, in view of pathological presence of MTB in samples and in cases of refractory adenitis ATT was given and had shown resolution [11-17].
Discussion
BCG lymphadenitis represents the predominant significant adverse event following BCG vaccination. In its natural course, BCG lymphadenitis can be classified into two types: suppurative BCG lymphadenitis, characterized by fluctuant swelling with erythema and edema of overlying skin and non suppurative lymphadenitis, which typically resolves spontaneously.
Clinical diagnosis remains the cornerstone of BCG lymphadenitis identification. The presence of isolated, swollen axillary or supraclavicular lymph nodes ipsilateral to the BCG vaccination site, without alternative explanation, typically suffices for diagnosis. The absence of constitutional symptoms distinguishes it from pyogenic adenitis [18-22].
Conventional investigations, including tuberculin testing, chest radiography and hematological analysis, generally provide limited diagnostic value. Current global estimates indicate annual BCG vaccination rates of approximately 100 million children. Mycobacterium bovis, which was used in the first BCG vaccination, is live attenuated. Almost 90% of vaccinations globally are carried out using four primary BCG vaccine strains: Tokyo strain 172, Glaxo strain 1077, Danish strain 1331 and Pasteur strain 1173 [23].
The majority of BCG-related mild side effects resolve on their own and don’t need medical attention. The BCG lymphadenitis suppurative type is regarded as a serious adverse effect. Recent advances in vaccination techniques and standardized protocols have contributed to declining rates of suppurative complications [24,25]
Treatment guidelines for BCG vaccine induced suppurative lymphadenitis are not well established. In literature, few authors recommend conservative care, such as medication and needle aspiration, while some active surgical treatment. There are many reports suggesting better results of surgical management. Many articles in the literature report ineffectiveness of medical treatment in suppurative lymphadenitis with fluctuations [26-28].
In our study, 50 patients presented with suppuration and required intervention immediately and other 4 eventually had suppuration requiring surgery. Documentation of cases of non-suppurative lymphadenitis could not be done as they did not visit to pediatric surgery OPD. Treatment protocol followed were consistent with avaliable literature. Only 4 cases who presented with ruptured abscess required incision and drainage with curettage. Surgical excision was required in cases where multiple aspirations were needed or nodes persisted despite conservative management. Surgical excision leads to faster healing in such cases [29,30].
Our study has potential limitations. Though we described 54 infants with BCG lymphadenitis, we could not comment on true incidence and trend as it was a department based study and we have no previous data to compare with. Notification of adverse effects to BCG vaccine is also not strictly followed. Duration of follow-up was also short to comment on complex response, recurrence and mortality.
Conclusion
Despite widespread BCG immunization in our country and adenitis being most common adverse effect of the vaccination, management strategies remain controversial. In our series, we have found that aspiration is effective treatment in most of the suppurative lymphadenitis, excision is a rescue procedure in cases refractory to multiple aspirations and in delayed cases which present with ulcer curettage is effective. We intend to contribute to the existing literature of this common but easily overlooked health problem.
References
- World Health Organization. Consolidated guidance on tuberculosis data generation and use. Module 1. Tuberculosis surveillance. World Health Organization; 2024.
- Zhang L, Ru HW, Chen FZ, Jin CY, Sun RF, Fan XY, et al. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Molecul Ther 2016;24(2):398-405.
[Crossref] [Google Scholar] [PubMed]
- Dhanawade SS, Kumbhar SG, Gore AD, Patil VN. Predictive factors for development of BCG lymphadenitis and its outcome: A prospective study. Indian J Pediatr 2023;90(5):445-450.
- Trunz BB, Fine PE, Dye CJ. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006;367(9517):1173-1180.
[Crossref] [Google Scholar] [PubMed]
- Roy P, Vekemans J, Clark A, Sanderson C, Harris RC, et al. Potential effect of age of BCG vaccination on global paediatric tuberculosis mortality: A modelling study. Lancet Glob Health 2019;7(12):1655-1663.
[Crossref] [Google Scholar] [PubMed]
- Martinez L, Cords O, Liu Q, Acuna-Villaorduna C, Bonnet M, Fox GJ, et al. Infant BCG vaccination and risk of pulmonary and extra pulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Global Health 2022;10(9):1307-1316.
[Crossref] [Google Scholar] [PubMed]
- Koeken VA, de Bree LC, Mourits VP, Moorlag SJ, Walk J, Cirovic B, et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest 2020;130(10):5591-5602.
[Crossref] [Google Scholar] [PubMed]
- Schaltz-Buchholzer F, Biering-Sørensen S, Lund N, Monteiro I, Umbasse P, Fisker AB, et al. Early BCG vaccination, hospitalizations, and hospital deaths: analysis of a secondary outcome in 3 randomized trials from Guinea-Bissau. J Infect Dis 2019;219(4):624-632.
[Crossref] [Google Scholar] [PubMed]
- Whittaker E, Nicol MP, Zar HJ, Tena-Coki NG, Kampmann B. Age-related waning of immune responses to BCG in healthy children supports the need for a booster dose of BCG in TB endemic countries. Sci Rep 2018;8(1):15309.
[Crossref] [Google Scholar] [PubMed]
- Al Jumaah S, Al Hajjar S, Al Mousa H. Bacille Calmette-Guerin vaccination in Saudi Arabia: Benefits versus risks. Ann Saudi Med 2012;32(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Chan WM, Kwan YW, Leung CW. Management of Bacillus Calmette-Guerin Lymphadenitis. Hong Kong J Paediat 2011;16(2):85-94.
- Milstien JB, Gibson JJ. Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ 1990;68(1):93.
[Google Scholar] [PubMed]
- Hengster P, Schnapka J, Fille M, Menardi G. Occurrence of suppurative lymphadenitis after a change of BCG vaccine. Arch Dis Child 1992;67(7):952-955.
[Crossref] [Google Scholar] [PubMed]
- Ladeira I, Carvalho I, Correia A, Carvalho A, Duarte R. BCGitis in children: BCGitis in children. Portuguese J Pulmonol 2014;20(3):172-173.
- Roy RB, Whittaker E, Kampmann B. Current understanding of the immune response to tuberculosis in children. Curr Opin Infect Dis 2012;25(3):250-257.
[Crossref] [Google Scholar] [PubMed]
- Singh AK, Gupta UD. Animal models of tuberculosis: Lesson learnt. Indian J Med Res 2018;147(5):456-463.
[Crossref] [Google Scholar] [PubMed]
- Moliva JI, Turner J, Torrelles JB. Immune responses to bacillus Calmette-Guerin vaccination: Why do they fail to protect against Mycobacterium tuberculosis?. Front Immunol 2017;8:407.
[Crossref] [Google Scholar] [PubMed]
- Mangtani P, Nguipdop-Djomo P, Keogh RH, Sterne JA, Abubakar I, Smith PG, et al. The duration of protection of school-aged BCG vaccination in England: A population based case control study. Int J Epidemiol 2018;47(1):193-201.
[Crossref] [Google Scholar] [PubMed]
- Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 2014;349.
[Crossref] [Google Scholar] [PubMed]
- Abubakar I, Pimpin L, Ariti CA, Beynon R, Mangtani P, Sterne JA, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol Assess 2013;17(37):1.
[Crossref] [Google Scholar] [PubMed]
- Marinova D, Gonzalo-Asensio J, Aguilo N, Martin C. Recent developments in tuberculosis vaccines. Expert Rev Vaccines 2013;12(12):1431-1448.
[Crossref] [Google Scholar] [PubMed]
- Daei Parizi M, Kardoust Parizi A, Izadipour S. Evaluating clinical course of BCG lymphadenitis and factors effect on it during a 5-year period in Kerman, Iran. J Trop Pediatr 2014;60(2):148-153.
- Govindarajan KK, Chai FY. BCG adenitis-need for increased awareness. Malaysian J Med Sci 2011;18(2):66.
[Google Scholar] [PubMed]
- Behjati M, Ayatollahi J. Post BCG lymphadenitis in vaccinated infants in Yazd, Iran. 2008.
- Singla A, Singh S, Goraya JS, Radhika S, Sharma M. The natural course of non-suppurative Calmette-Guerin bacillus lymphadenitis. Pediatric Infect Disease J 2002;21(5):446-448.
[Crossref] [Google Scholar] [PubMed]
- Banani SA, Alborzi A. Needle aspiration for suppurative post-BCG adenitis. Arch Dis Child 1994;71(5):446-447.
[Crossref] [Google Scholar] [PubMed]
- Engelis A, Kakar M, MeikšÄns R, Petersons A. BCG-SSI® vaccine-associated lymphadenitis: Incidence and management. Medicine 2016;52(3):187-191.
[Crossref] [Google Scholar] [PubMed]
- Soh SB, Han PY, Tam KT, Yung CF, Liew WK, Tan NW, et al. Investigations into an outbreak of suppurative lymphadenitis with BCG vaccine SSI® in Singapore. Vaccine 2014;32(44):5809-5815.
[Crossref] [Google Scholar] [PubMed]
- Hengster P, Sölder B, Fille M, Menardi G. Surgical treatment of bacillus Calmette Guerin lymphadenitis. World J Surg 1997;21(5):520-523.
[Crossref] [Google Scholar] [PubMed]
- Cuelloâ?García CA, Pérezâ?Gaxiola G, Gutiérrez CJ. Treating BCGâ?induced disease in children. Cochrane Database Syst Rev 2013(1).
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 The Authors. This is an open access article under the terms of the Creative Commons Attribution Non Commercial Share Alike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.