Case Report - Archives of Clinical and Experimental Surgery (2022)
Comprehensive Treatment of a Rare Primary Malignant Pericardial Mesothelioma: A Case Report and Literature Review
Chunyu Liu, Bin Zhao, Weiwei Cheng, Dongliang Ma, Xing Wei and Shunye Zhang*Shunye Zhang, Department of Cardiac Surgery, Shanxi Cardiovascular Hospital, Taiyuan of Shanxi province, China, Tel: +8613663618362, Email: xxgwk@139.com
Received: 21-Jul-2022, Manuscript No. EJMACES-22-69791; Editor assigned: 25-Jul-2022, Pre QC No. EJMACES-22-69791 (PQ); Reviewed: 08-Aug-2022, QC No. EJMACES-22-69791; Revised: 16-Aug-2022, Manuscript No. EJMACES-22-69791 (R); Published: 23-Aug-2022
Abstract
Background: Primary Malignant Pericardial Mesothelioma (PMPM) is an extremely rare aggressive malignancy with nonspecific symptoms; thus, cases of PMPM are usually found incidentally. Despite treatment, it is considered as extremely fatal.
Case presentation: We encountered an asymptomatic 64-year-old woman diagnosed with PMPM by Positron Emission Tomography/Computed Tomography (PET/CT). After 22 months of supportive treatment and chemotherapy, she presented to our facility with progressive dyspnoea. Surgery was performed. Excision biopsy showed thickening pericardium and many poorly circumscribed masses, and cut sections showed friable grey–white tissue with areas of necrosis. Pathological examination confirmed epithelium-like malignant mesothelioma. The patient was continuously treated with a previously prescribed chemotherapy regimen. The patient did not exhibit dyspnoea recurrence, and PET/CT showed no progression 7 months postoperatively.
Conclusion: Comprehensive treatment, including chemotherapy, supportive treatment, and timely surgery, is effective for unresectable PMPM. When patients present with symptoms caused by PMPM that are not relieved by medication, we recommend prioritising urgent surgery.
Keywords
Primary malignant pericardial mesothelioma; Comprehensive treatment; Diagnosis; Case report
Abbreviations
MSCT: Multislice Computed Tomography; CMR: Cardiac Magnetic Resonance; PET/CT: Positron Emission Tomography/ Computed Tomography; TTE: Trans Thoracic Echocardiography; PMPM: Primary Malignant Pericardial Mesothelioma
Introduction
Cardiac tumours are rare [1]. Pericardial malignancies account for only 0.0025% of all malignant tumours and less than 2% of all cardiac tumours [2,3]. PMPM, originating from pericardial mesothelial cells, can be divided into diffuse and localised types. The diffuse type is characterised by obvious bleeding, exudation, and infiltrative growth, especially along the pericardium. In the early stage, PMPM can only show nodule-like, plaque-like or warty protrusions on the pericardial surface, and then the pericardium gradually thickens. In the late stage, part or even all of the heart is surrounded by the tumour, resulting in symptoms of cardiac compression. PMPM invades the heart or other organs owing to its insidious nature and atypical symptoms. PMPM has a poor prognosis because it is difficult to treat with radical surgical resection, and there is insufficient evidence regarding the effects of radiotherapy and chemotherapy on this disease entity. Thomason et al. presented a case report and review of PMPM, and Basso provided recommendations for its treatment, but standardized guidelines for the treatment of PMPM are lacking.
Case Presentation and Therapy
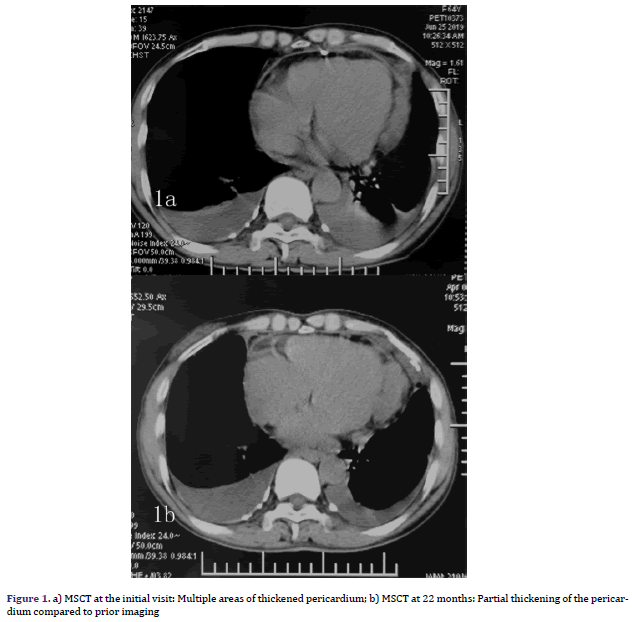
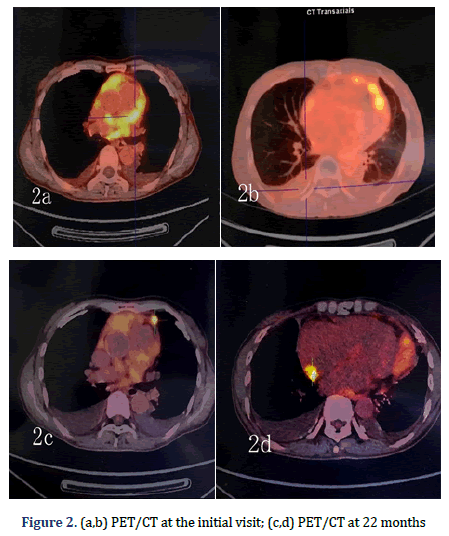
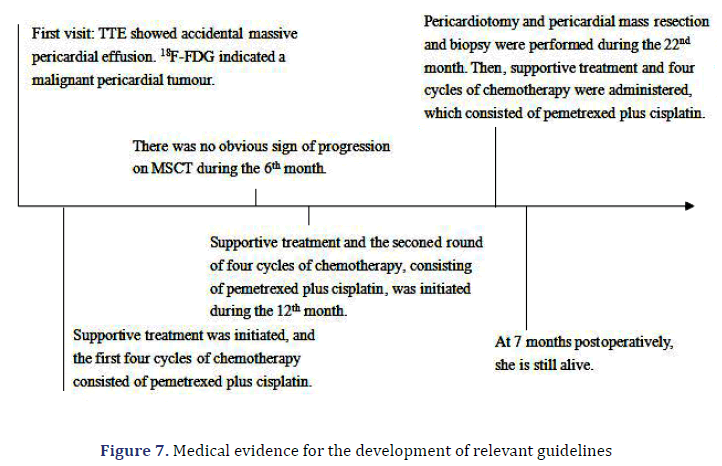
A 64-year-old female patient with an unremarkable medical history, except for cholecystectomy for cholecystitis with gallstones 7 years prior, presented to our facility with asymptomatic massive pericardial effusion accidentally found through Trans Thoracic Echocardiography (TTE). Multi Slice Computed Tomography (MSCT) and CMR showed moderate pleural effusion on both sides (further laboratory tests revealed that the Rivalta reaction was weakly positive, and tumour markers were negative) and multiple thickened pericardium (Figures 1a and 1b). 18F-FDG imaging-Position Emission Tomography/ Computed Tomography (PET/CT) showed scattered a partial increase in pericardial metabolism (SUVmax= 5.75-6.82) but no distant metastasis (Figures 2a-2d). A malignant pericardial tumour was suspected and appeared to be completely unresectable. For further diagnosis, ultrasound-guided pericardiocentesis was performed, and laboratory analysis revealed that adenosine deaminase tests were negative, and no tumour markers were found, which may be related to too few punctured tissues or the poor diagnostic effect of pericardial effusion on malignant pericardial tumours. Pericardial effusion did not recur after pericardiocentesis. After multidisciplinary discussions, she received supportive treatment and chemotherapy with pemetrexed plus cisplatin for 21 days for four cycles. During the first cycle of chemotherapy, the patient presented with nausea and platelet levels of <50000 cell/mm3 after the administration of pemetrexed (500 mg/m2) plus cisplatin (75 mg/m2). For the second cycle, we reduced the dose of pemetrexed to 250 mg/m2 and the dose of cisplatin to 37.5 mg/m2. She tolerated this reduced dose and completed all four cycles of chemotherapy. However, her platelet count was still low, even though she received best supportive treatment. She continued to receive best supportive treatment, and we re-evaluated her via MSCT and TTE every six months. No tumour progression was noted on MSCT at the 6-month follow-up. However, it did reveal that the tumour had grown during the 12th month. A second four-cycle round of chemotherapy was recommended. However, her performance status deteriorated gradually at the end of the first cycle of the second round, and she was unable to receive additional palliative chemotherapy. Oncologists and nutritionists recommend the suspension of chemotherapy while maintaining comprehensive best supportive care. The nutritional status of the patient improved significantly, but her pericardial disease was found to have progressed at the 22nd month of follow-up.
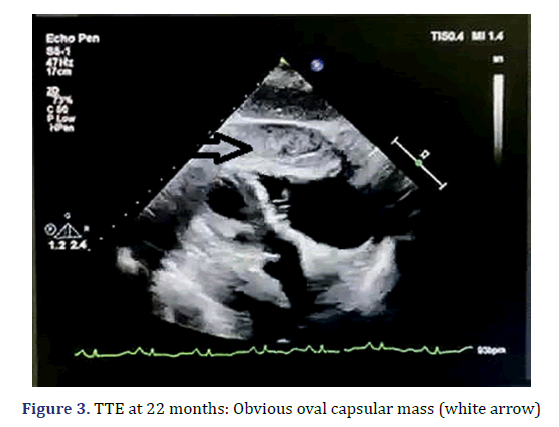
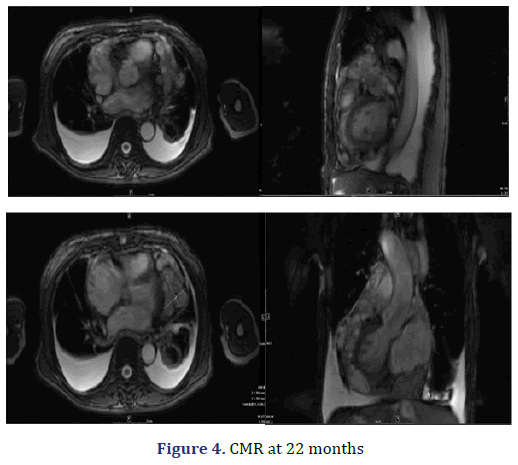
The patient presented to our facility with progressive dyspnoea but no orthopnoea, chest pain, or other forms of discomfort in the 22nd month. On clinical examination, her heart rate was 130 bpm, and her respiratory rate was 30 breaths/min with an oxygen saturation of 95% on room air. Her blood pressure was 100/60 mmHg with evidence of pulsus paradoxus, and her central venous pressure was 14 cm H2O. In addition, she had bilateral congested neck veins and distant heart sounds. Most serologic tests were within the normal range. ECG showed low voltage and sinus tachycardia. TTE revealed a thickened pericardium and a noticeable oval capsule mass compressing the left ventricle throughout the entire cardiac cycle (Figure 3). MSCT and CMR revealed partial thickening of the pericardium comprised of diffuse masses of varying sizes (Figure 1b, Figure 4). Re-examination by PET/CT showed that metabolism of some of the masses had significantly increased, while others had not, but the range was significantly increased compared with that from 22 months prior; no distant metastasis was observed (Figures 2c and 2d).
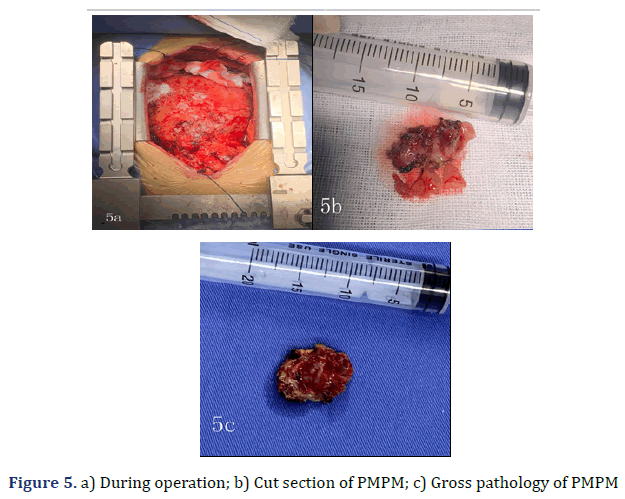
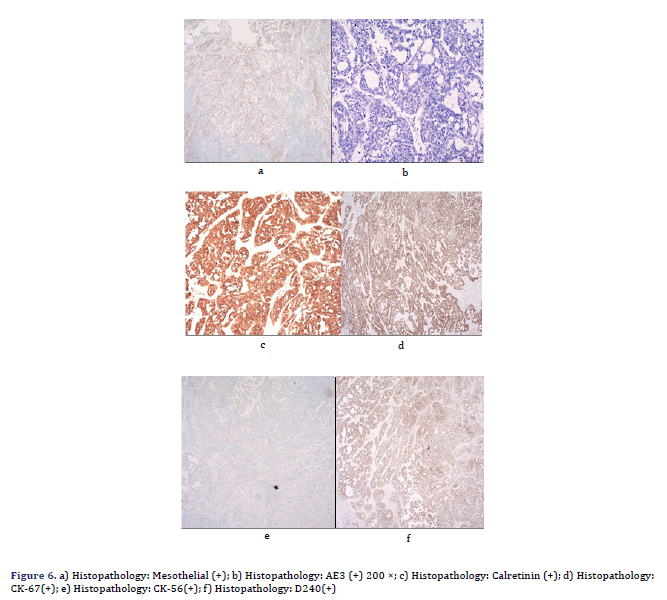
Her pleural effusion increased significantly, and her dyspnoea symptoms were not relieved, even after closed thoracic drainage. Thus, pericardiotomy and pericardial mass resection and biopsy were subsequently performed (Figure 5). Some masses were observed to surround the aorta and pulmonary artery and involved the left ventricle [4]. These masses could be resected to a certain point, even if they could not be completely resected. Therefore, partial resection of the anterior pericardium was performed. The pericardium at the junction of the superior vena cava and the right atrium was partially removed to loosen the constriction of the mesothelium. Gross pathology of some of the masses revealed a well-defined encapsulated tumour, and the cut section showed friable grey–white tissue with areas of chocolate-like necrosis (Figure 5). The histopathology showed epithelium-like malignant mesothelioma, AE1/AE3(+), Calretinin(+), D240(+), CK56(partial+), CK7(+), mesothelial(+), Vimentin(partial cells+), WT-1(-), MOC-31(-), Ber-EP4(-), TTF-1(-), Napsin A(-), P53(partial+), PAX-8(-), CK19(+), CK20(- ), ER(-), PR(-), and Ki67(approximately 60%+) (Figure 6). Histologic sectioning confirmed that the tumour was PMPM. After surgery, the patient’s central venous pressure decreased to 3 cm H2O, and her blood pressure increased and remained stable at 110/80 mmHg. We present preliminary data from a case report to provide specific medical evidence for the development of relevant guidelines (Figure 7).
Outcome and follow-up
After the surgery, the patient was administered conventional cardiotonic, diuretic and other symptomatic treatments. Her overall condition had improved, and TTE evaluation showed that her cardiac ejection fraction was 60%. The patient then continued the previous chemotherapy regimen, pemetrexed at a dose of 125 mg/m2 plus cisplatin at a dose of 18.75 mg/m2 for four cycles. Follow-up PET/CT did not show any recurrence or distant metastasis at 7 months postoperatively. Further follow-up will continue.
Results and Discussion
In clinical work, various questions arise for surgeons: What kind of imaging examination should be used to make an early and accurate diagnosis? Which treatment is better? What surgical treatment will confer the most benefit to the patient? The latest guidelines do not include specific recommendations for malignant pericardial tumours [5]. Thus, this paper describes the diagnosis and treatment of a case of PMPM to provide guidance for clinicians in making decisions regarding treatment planning.
It is challenging to diagnose pericardial tumours using only the patient’s medical history, clinical signs, cardiac X-ray and TTE. TTE, MSCT and CMR have their own unique advantages and disadvantages [6], but diagnosis still depends on histopathology. PMPM patients do not present dyspnoea—the most common symptom—or other nonspecific symptoms when massive pericardial effusion and pericardial thickening are occasionally found by TTE. Because MSCT and CMR do not provide any more discriminating information than TTE, PET/ CT was performed, which indicated the possibility of a malignant pericardial tumour. This cautiously suggests that when the patient is suspected of pericardial malignancy but does not accept or cannot undergo histopathological examination, the use of PET/CT may aid in making an accurate diagnosis [7,8].
In 1997, Basso et al. pointed out that surgery is the first and only choice for PMPM [9]. The recent cases in the literature provided evidence to support the use of combined therapy—pericardiectomy-assisted radiotherapy and/or chemotherapy for patients with limited PMPM and pericardial perfusion chemotherapy after fenestration and drainage for patients with diffuse PMPM—in patients who do not meet the criteria for complete resection. This combination therapy can alleviate pain and prolong the survival time [10]. Despite many new advances in the diagnosis and treatment of PMPM, its prognosis is dismal, with a median survival time of six months [11,12]. Standard treatment for diffuse PMPM has not been well established. It has been reported that pemetrexed plus cisplatin, the only approved first-line treatment for patients with unresectable malignant pleural mesothelioma, is also effective in the short term for localised PMPM [13]. At the time of accidental discovery, the tumour was diffuse and could not be completely resected. Thus, chemotherapy and supportive treatment were provided. Due to manifestations of haematotoxicity and gastrointestinal toxicity, the dosage of the chemotherapy regimen was adjusted twice. After one and one-quarter of four cycles of chemotherapy, owing to the poor nutritional status and the reoccurrence of haematotoxicity and gastrointestinal toxicity, chemotherapy was suspended, and the patients received best supportive treatment. After 10 months of supportive treatment, patient’s general condition had improved, but she suffered disease progression, and her condition was similar to constrictive pericarditis. Excluding pleural effusion, it was determined that her symptoms were caused by a narrowed pericardium. Surgery was performed promptly, and symptoms were significantly relieved. The patient’s cardiac ejection function was 60% after surgery, and the previous chemotherapy regimen was resumed. The patient was treated with comprehensive treatment for 22 months before the operation. After 7 months postoperatively, she remains alive and continues to receive best supportive care 29 months after the first clinical manifestation. This proves that our treatment is effective.
In 1994, Thomason et al. presented a case report and reviewed 27 cases of primary pericardial mesothelioma that appeared in English literature from 1972 to 1992 [14]. Ase Nilsson, et al. reviewed 29 cases reported in the English literature from 1993 through 2008 in which a diagnosis of primary pericardial mesothelioma was established. In the available literature, we found twelve cases of PMPM (four males and eight females) diagnosed between 2009 and 2020 (Table 1). The mean age at the time of diagnosis was 47.92 ± 14.15 years. Dyspnoea was the most common clinical symptom, which was reported in 12 cases (8/12, 66.67%). We did not find any patients with a history of asbestos exposure among these cases, consistent with the current mainstream opinion. The histologic type of 2 patients was not mentioned; 4 were the biphasic type (33.33%) and 6 were the epithelial type (50.00%). Seven of the 12 cases were treated with chemotherapy in combination with surgery; the longest survival time was 27 months, and the shortest survival time was 3 months.
| No. | Author, year |
Age, gender |
Symptoms | Asbestosis | Diagnosis methods |
Tumor size(cm) | Metastases and invansion | Histologic type | Treatments | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ase Nilsson, 2009 | 38,M | Chest pain, dyspnea | No | TTE,CMI,PET/CT | 5 × 4 × 2.5 | No | Biphasic | Surgery, Pemetrexed+cispatin |
15 mo without symptoms |
| 2 | Kelli Reardon, 2010 | 59,FM | Chest pain, dyspnea | No | X-rary, CT,CMI | 7.4 × 4.8 × 4.2 | Adherence to right ventricle | Biphasic | PericardialWindows,gemcitabine+cisplatin,gemcitabine+carboplatin.permextred,Radiation | 50 mo without progression |
| 3 | Mohit Godar, 2013 | 68,M | Dyspnea | No | TTE,CT | 3.5 | No | Epithelial | Surgery, Pemetrexed+cispatin |
Died after 27 mo |
| 4 | Weihui Gong, 2014 | 60,FM | Dry cough, shortness of breath | No | TTE,CT,PET/CT | 11 × 9 × 2 | Adherence to right ventricle | Epithelial | Pericardiocentesis,Thoracentesis,Surgery,cisplatin+eoposide | Died after 3 mo |
| 5 | Russell Fernandes, 2014 | 30,FM | No | No | TTE,CT,CMI | 10.2 × 5.2 × 0.6 | Adherence to right ventricle | Epithelial | Surgery | Died |
| 6 | RajooRamachandran, 2014 | 55,FM | Dyspnea | No | TTE,CT,PET/CT | Max. thickeniess of 1.5 | No | Biphasic | CT-guided biopsy | Not mention |
| 7 | Takeyukikurosawa, 2016 | 37.FM | Chest pain, dyspnea | No | TTE,CT,PET/CT | Irregular,thickened pericardium | Adherence to right ventricle | Epithelial | Surgery,carboplatin+pemetrexed | Died after 18 mo |
| 8 | SANG MI CHUNG, 2016 | 52,FM | Dyspnea, coughing, fever | No | X-rary, TTE,CT,CMI | surrounding the heart | Metastased to both lung fields | Epithelial | Pericardial biopsy,pericardiocentesis,Surgery,Pemetrexed+cispatin | 21 mo whitout symptoms |
| 9 | Xiaohui Li,2017 | 28,M | chest pain, Shortness of breath | No | X-rary, TTE,CT,PET/CT | surrounding the heart | Bone and right atrium etastasis | Not mention | Pericardial biopsy, Surgery,Pemetrexed+cispatin | Died after 27 mo |
| 10 | Kohei Shikano, 2018 | 64,FM | Dyspnea | No | X-rary, TTE,CT | surrounding the heart | Invasion of myocardium, right pleura and lung | Epithelial | Pericardiocentesis,VATS-pericardial fenestration,surgery | Died after 5 mo |
| 11 | Guang Song,2019 | 52,M | Chest pain, dyspnea | No | TTE,CT,PET/CT,CMI | 5 × 4 × 2.5 | No | Biphasic | Pericardiocentesis, surgery | Still alive after 29 mo |
| 12 | Sameh Ben Farhat, 2020 | 32,FM | Shortness of breath | No | X-ray, TTE,CT | surrounding the heart | No | Not mention | Pericardiocentesis,surgery,chemo+radiotherapy | Died after 4 mo |
| Note: CT: Computed Tomography; F: Female; M: Male; CMR- Cardiac Magnetic Resonance; PET/CT: Positron Emission Tomography/Computed Tomography; TTE: Transthoracic | ||||||||||
Conclusion
In conclusion, comprehensive treatment, including chemotherapy and best supportive treatment, and timely surgery is effective for unrespectable PMPM. When patients present with symptoms caused by PMPM that are not relieved by medication, we recommend prioritising urgent surgery. There were no patients found with history of asbestosis. All the patients had a common symptom of dysphonia (i.e shortness in breathing). After proper examinations, With respect to the patient’s condition treatment was undergone.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests in financial and non-financial.
Funding
Not applicable.
Authors’ contributions
All authors contributed equally within the limits and beyond their speciality in accumulating knowledge, diagnosing and managing this rare condition and have approved the manuscript. CYL and BZ were involved in disease management and follow-up of the patient. CYL was involved in the thorough literature review. WWC was involved in the radiological investigations throughout the follow-up. DLM was involved in the histological evaluation of the tumour. XW was involved in the surgical management and follow up of the patient. SYZ revised the manuscript critically for important intellectual content and gave final approval for this manuscript version to be submitted.
Acknowledgements
We thank Lin Wang, Pathologist of Shanxi Bethune Hospital, for her professional help.
References
- Fernandes R, Nosib S, Thomson D, Baniak N. A rare cause of heart failure with preserved ejection fraction: primary pericardial mesothelioma masquerading as pericardial constriction. BMJ Case Rep 2014; 2014: bcr2013203194.
[Crossref] [Google Scholar] [Pubmed]
- Song G, Bi W, Zhang X, Huang W, Zhou K, Ren W, et al. Localized primary malignant pericardial mesothelioma. J Clin Ultrasound 2019; 47(3):178-181.
[Crossref] [Google Scholar] [Pubmed]
- Papi M, Genestreti G, Tassinari D, Lorenzini P, Serra S, Ricci M, et al. Malignant pericardial mesothelioma. Report of two cases, review of the literature and differential diagnosis. Tumori 2005;91(3):276-9.
[Crossref] [Google Scholar] [Pubmed]
- Apicella G, Boulemden A, Citarella A, Sushma R, Szafranek A. Surgical treatment of a primary malignant pericardial mesothelioma: Case report. Acta Chirurgica Belgica 2022; 122(1):48-50.
[Crossref] [Google Scholar] [Pubmed]
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the Diagnosis and Treatment of Pericardial Diseases. Kardiol Pol 2015;73(11):1028-1091.
[Crossref] [Google Scholar] [Pubmed]
- Chung SM, Choi SJ, Kim MJ, Choi JY, Kim HJ, Lee SY, et al. Positive response of a primary malignant pericardial mesothelioma to pemetrexed plus cisplatin followed by pemetrexed maintenance chemotherapy: A case report. Oncol Lett 2016;12(1):213-6.
[Crossref] [Google Scholar] [Pubmed]
- Kurosawa T, Sugino K, Isobe K, Hata Y, Fukasawa Y, Homma S, et al. Primary malignant pericardial mesothelioma with increased serum mesothelin diagnosed by surgical pericardial resection: A case report. Mol Clin Oncol 2016;5(5):553-6.
[Crossref] [Google Scholar] [Pubmed]
- Hollevoet K, Reitsma JB, Creaney J, Grigoriu BD, Robinson BW, Scherpereel A, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol 2012; 30(13):1541-9.
[Crossref] [Google Scholar] [Pubmed]
- Basso C, Valente M, Poletti A, Casarotto D, Thiene G. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg 1997;12(5):730-8.
[Crossref] [Google Scholar] [Pubmed]
- Maruyama R, Sakai M, Nakamura T, Suemitsu R, Okamoto T, Wataya H, et al. Triplet chemotherapy for malignant pericardial mesothelioma: A case report. Jpn J Clin Oncol 2006; 36(4):245-8.
[Crossref] [Google Scholar] [Pubmed]
- Shimazaki H, Aida S, Iizuka Y, Yoshizu H, Tamaj S. Vacuolated cell mesothelioma of the pericardium resembling liposarcoma: A case report. Hum Pathol 2000;31(6):767-70.
[Crossref] [Google Scholar] [Pubmed]
- Nilsson A, Rasmuson T. Primary pericardial mesothelioma: Report of a patient and literature review. Case Rep Oncol 2009;2(2):125-32.
[Crossref] [Google Scholar] [Pubmed]
- Kim JS, Lim SY, Hwang J, Kang EJ, Choi YJ. A Case Report of Primary Pericardial Malignant Mesothelioma Treated with Pemetrexed and Cisplatin. J Korean Med Sci 2017;32(11):1879-84.
[Crossref] [Google Scholar] [Pubmed]
- Thomason R, Schlegel W, Lucca M, Cummings S, Lee S. Primary malignant mesothelioma of the pericardium. Case report and literature review. Tex Heart Inst J 1994;21(2):170-174.
[Crossref] [Google Scholar] [Pubmed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.