Research Article - Archives of Clinical and Experimental Surgery (2023)
Mitral Valve Repair Improves Long-Term Cardiac Functional Outcome in Patients with Infective Endocarditis
Manabu Shiraishi*, Kengo Teshima, Hiroki Arai, Shigeto Tokunaga, Naoyuki Kimura and Atsushi YamaguchiManabu Shiraishi, Department of Cardiovascular Surgery, Saitama Medical Center, Jichi Medical University, Saitama, Japan, Email: manabu@omiya.jichi.ac.jp
Received: 25-Sep-2023, Manuscript No. EJMACES-23-114638; Editor assigned: 27-Sep-2023, Pre QC No. EJMACES-23-114638 (PQ); Reviewed: 11-Oct-2023, QC No. EJMACES-23-114638; Revised: 18-Oct-2023, Manuscript No. EJMACES-23-114638 (R); Published: 25-Oct-2023
Abstract
Objectives: The aim of the investigation was to compare long-term clinical and echocardiographic outcomes of Mitral Valve Repair (MVRep) against those of Mitral Valve Replacement (MVR) performed for Infective Endocarditis (IE).
Background: Several observational studies have suggested better survival after performance of MVRep vs. MVR in patients with IE. However, factors affecting the feasibility of MVRep and its effects on late-period cardiac function remain unknown.
Methods: This retrospective study included 101 consecutive patients referred to our institution between April 1990 and December 2022 and treated for mitral valve IE (63 by MVR and 38 by MVRep). Perioperative variables and long-term outcomes were compared between the 2 patient groups.
Results: Active IE, heart failure and a large area of Leaflet destruction were found to be independent predictive factors for selection of MVRep. In-hospital death occurred in 2 (2.0%) cases (2 MVR group patients), and 12 (11.9%) patients (11 MVR group patients and MVRep group patient) died during follow-up. Higher 10-year survival (94.7% vs. 75.2%) and event-free 10-year survival (72.2% vs. 66.8%) were observed in the MVR group vs. the MVRep group). In addition, re-intervention (7.9% vs. 21.1%), MR recurrence (13.2% vs. 21.1%) and Atrial Fibrillation (AF) (5.3% vs. 33.3%) rates were lower following MVRep. Echocardiographic follow-up revealed significant reverse remodeling and an improved trans-mitral pressure gradient in the MVRep group.
Conclusion: MVRep for mitral valve IE is feasible and yields good perioperative outcomes. The procedure appears to suppress AF by effecting significant reductions in left ventricular dimensions and left atrial load.
Keywords
Infective endocarditis; Mitral valve repair; Mitral valve replacement; Cardiac function; Atrial fibrillation
Introduction
Mitral Valve Repair (MVRep) is considered the ideal treatment for degenerative Mitral Regurgitation (MR) and improved techniques have made it possible to repair complex valve defects. Even for Infective Endocarditis (IE), a life-threatening disease with high mortality, previous studies reported that MVRep leads to better outcomes compared to Mitral Valve Replacement (MVR), also showing that MVRep can be performed in most cases of active IE [1,2]. MVRep is advantageous in terms of decreased perioperative mortality and improved survival, fewer anticoagulant-related complications and recurrences of the IE and preserved left ventricular function [3-5]. However, there are concerns about the durability of complex MVRep, particularly when performed on infected tissue in cases of active IE [6]. MVR remains an important treatment, especially in cases of severe valvular destruction and 1 or more large vegetations [7,8]. Despite indications of the superiority of MVRep, minimal data are available regarding outcomes of MVRep in terms of cardiac function and arrhythmic events, minimal data are available regarding outcomes of MVRep. Specifically, it is unclear whether MVRep for IE, in comparison to MVRep for degenerative MR, prevents left ventricular dilation and induces reverse remodeling in the long term. We conducted a retrospective, single-center study to identify factors influencing the selection of MVR or MVRep in patients with IE affecting the mitral valve, to identify independent predictors of postoperative mortality, reintervention and recurrence of MR and to evaluate cardiac function in the long term.
Materials and Methods
Patients
The study was approved by the Institutional Review Board of Jichi Medical University (Approval no. S22-102). Informed consent was secured through an opt-out system available to patients on the institution’s website. Preoperative and postoperative outcome variables were extracted from our institutions’ adult cardiac surgery database. Patients included in the study (59 men (58.4%) and 42 women (41.6%), aged 20 years or more) were identified from among a total of 279 consecutive patients who, between April 1990 and December 2022, had undergone surgery for IE affecting the mitral valve. The IE had been diagnosed in the 101 study patients according to the modified Duke criteria [9]. Mean age of the study patients was 59.4 ± 15.7 years. Patients were divided into 2 groups; an MVR group (n=63) and an MVRep group (n=38).
Patients’ preoperative characteristics are summarized per group in Table 1. There was no significant difference between the 2 groups in age, sex, or medical history. A significantly greater proportion of patients in the MVR group (vs. the MVRep group) were of New York Heart Association (NYHA) functional class III or IV (P=0.0359). Overall, Streptococcus was the most common causative microorganism (n=40), followed by Staphylococcus (n=16), with no significant between-group difference in the incidence of either of these 2 causes. Treatment was based on the results of drug susceptibility testing. Eighty-two of the total patients were treated for active IE and 19 for healed IE, with the IE judged to be active on the basis of positive preoperative or intraoperative blood cultures, continued antibiotic therapy since the initial diagnosis, positive tissue culture or a positive pathology report and notice of obvious vegetation during the surgery. The following were considered indications for surgery in patients with active IE: Heart failure, uncontrolled sepsis, a systemic embolic event, mobile vegetation and severe MR due to valve destruction. There was no significant between-group difference in the time from diagnosis to surgery in cases of active IE.
| MVR group (n=63) | MVRep group (n=38) | P value | |
|---|---|---|---|
| Age (years) | 60.7 ± 15.7 | 57.2 ± 15.6 | 0.2777 |
| Age>70 years | 20 (31.7) | 9 (23.7) | 0.4971 |
| Sex, male | 34 (54.0) | 25 (65.8) | 0.2993 |
| Medical history | |||
| Hypertension | 17 (27.0) | 11 (28.9) | 0.8231 |
| Dyslipidemia | 7 (11.1) | 2 (5.3) | 0.4771 |
| Diabetes mellitus | 8 (12.7) | 3 (7.9) | 0.5285 |
| Renal dysfunction (Cr>1.5 mg/dL) | 5 (7.9) | 2 (5.3) | 0.7078 |
| COPD | 0 (0.0) | 0 (0.0) | >0.9999 |
| Previous cardiac surgery | 8 (12.7) | 3 (7.9) | 0.5285 |
| NYHA functional class | |||
| I or II | 44 (69.8) | 30 (78.9) | 0.3609 |
| III or IV | 16 (25.4) | 3 (7.9) | 0.0359 |
| Causative organisms of IE | |||
| Genus Streptococcus | 26 (41.3) | 14 (36.8) | 0.6808 |
| Genus Staphylococcus | 13 (20.6) | 3 (7.9) | 0.1013 |
| Staphylococcus aureus | 11 (17.5) | 3 (7.9) | 0.24 |
| Genus Enterococcus | 2 (3.2) | 3 (7.9) | 0.3618 |
| Other | 2 (3.2) | 3 (7.9) | 0.3618 |
| Culture negative | 19 (30.2) | 7 (18.4) | 0.243 |
| Stage of endocarditis | |||
| Active | 60 (95.2) | 22 (57.9) | 0.0004 |
| Healed | 3 (4.8) | 16 (42.1) | 0.0004 |
| Indication(s) for MVR/MVRep | |||
| Heart failure | 26 (41.3) | 7 (18.4) | 0.0277 |
| Uncontrolled sepsis | 13 (20.6) | 1 (2.6) | 0.0016 |
| Systemic embolic event | 20 (31.7) | 3 (7.9) | 0.0065 |
| Mobile vegetation | 25 (39.7) | 8 (21.1) | 0.0079 |
| Severe mitral regurgitation | 45 (71.4) | 29 (76.3) | 0.6485 |
| Time from diagnosis to surgery (days) | 9.6 ± 9.9 | 7.6 ± 11.0 | 0.1494 |
Note: Values are mean ± SD or n (%); COPD: Chronic Obstructive Pulmonary Disease; Cr: Serum Creatinine; IE: Infective Endocarditis; NYHA: New York Heart Association.
Note:
Table 1. Patients’ preoperative characteristics, per study group.
Echocardiography
Trans Thoracic 2-dimensional Echocardiography (TTE) at rest was performed preoperatively, as previously described and anatomic features, the degree of valve tissue destruction and paravalvular extension of infection were thus evaluated [10]. TTE was also performed in the early postoperative period (up to 4 weeks after the surgery) and in the late postoperative period. The mean follow-up period was 57 months (range: 1-306 months). In addition, differences between preoperative and postoperative cardiac variables were evaluated in each group. MR was characterized as mild=1+(jet area/left atrial area<10%), moderate=2+(jet area/left atrial area 10-20%), moderate-severe=3+(jet area/left atrial area 20-45%), or severe=4+(jet area/left atrial area >45%) [11]. Investigators were blinded to patients’ clinical information and all echocardiographic data were analyzed by 3 experienced cardiologists. Preoperative cardiac variables did not differ significantly between the 2 groups (Table 2).
| MVR group (n=63) | MVRep group (n=38) | P value | |
|---|---|---|---|
| LAD (mm) | 48.6 ± 10.0 | 47.9 ± 10.1 | 0.6511 |
| LVDd (mm) | 53.7 ± 6.9 | 55.3 ± 6.9 | 0.4667 |
| LVDs (mm) | 33.9 ± 5.9 | 34.9 ± 6.2 | 0.5976 |
| LVEF (%) | 66.2 ± 7.8 | 65.7 ± 8.0 | 0.8015 |
| TR-PG (mmHg) | 34.7 ± 16.4 | 23.1 ± 8.3 | 0.0002 |
| E/e' | 25.7 ± 14.3 | 18.1 ± 8.0 | 0.0444 |
| MV peak v (m/s) | 1.9 ± 0.7 | 2.3 ± 0.1 | 0.0879 |
| MV max PG (mmHg) | 17.2 ± 15.5 | 16.8 ± 8.2 | 0.4308 |
| MV mean PG (mmHg) | 4.8 ± 2.6 | 6.3 ± 3.7 | 0.5549 |
Note: Values are mean ± SD; LAD: Left Atrial Dimension; LVDd: Left Ventricular End-Diastolic Dimension; LVDs: Left Ventricular End-Systolic Dimension; LVEDV: Left Ventricular End-Diastolic Volume; LVEF: Left Ventricular Ejection Fraction; LVESV: Left Ventricular End-Systolic Volume; MV: Mitral Valve; SV: Systolic Volume; TR-PG: Tricuspid Regurgitation Pressure Gradient.
Note: 
Surgical procedures
All surgeries, whether MVRep or MVR, were performed by experienced surgeons in a consultative capacity. On the technical side, the first step was radical debridement of infectious material and the second step was morphologic and functional mitral valve reconstruction. The main pathologies observed at the time of surgery are shown in Table 3. MVR was performed significantly more frequently for obvious vegetation, extensive leaflet destruction and/or anterior leaflet prolapse, whereas MVRep was performed significantly more frequently for chordae rupture and/or posterior leaflet prolapse. Details of the surgical procedures are shown in Table 4. Mean operation time, mean aortic cross-clamp time and mean cardiopulmonary bypass time did not differ between the 2 groups. If durable MVRep was considered technically infeasible, MVR was initiated. If MVRep failed (MR remaining above grade 2 on intraoperative echocardiography), MVR was undertaken intraoperatively. For MVR, the chordae tendineae-sparing technique was used to prevent postoperative loss of left ventricular function. In the performance of MVRep, all infected tissue was first removed. The surgical techniques were based on the MVRep strategies proposed by Carpentier [1,12]. MVRep consisted, in principle of preservation or restoration of normal valve leaflet motion, securing a wide coaptation zone and stabilization of the mitral annulus. Trans Esophageal 2-dimensional Echocardiography (TEE) was performed immediately after the surgery to assess residual MR; the maximum regurgitant jet area and length measured on TEE was used to determine whether the MVR or MVRep was successful. The MVR or MVRep was combined with aortic valve replacement for IE affecting the aortic valve in 1 patient and with tricuspid valve repair in 35 patients. All patients operated on for active IE continued antibiotic therapy for 6 weeks following the surgery.
| MVR group (n=63) | MVRep group (n=38) | P value | |
|---|---|---|---|
| Vegetation | 46 (73.0) | 16 (42.1) | 0.003 |
| Perforation | 12 (19.0) | 10 (26.3) | 0.4585 |
| Chordae rupture | 23 (36.5) | 26 (68.4) | 0.0022 |
| Large area of leaflet destruction | 22 (34.9) | 0 (0.0) | <0.0001 |
| Valve prolapse | |||
| Posterior leaflet | 13 (20.6) | 23 (60.5) | <0.0001 |
| Anterior leaflet | 22 (34.9) | 6 (15.8) | 0.0418 |
| Both leaflets | 3 (4.8) | 0 (0.0) | 0.2889 |
| Annular abscess | 4 (6.3) | 1 (2.6) | 0.6476 |
Note: Values are n (%).
| MVR group (n=63) | MVRep group (n=38) | P value | |
|---|---|---|---|
| Operation time (min) | 304.2 ± 87.3 | 278.2 ± 43.2 | 0.2611 |
| Aortic cross-clamp time (min) | 112.1 ± 39.3 | 112.4 ± 27.4 | 0.5783 |
| Cardiopulmonary bypass time (min) | 138.2 ± 49.5 | 136.7 ± 28.2 | 0.5008 |
| Surgical procedure | |||
| Mechanical valve | 40 (63.5) | N/A | |
| Bioprosthetic valve | 23 (36.5) | N/A | |
| Primary closure for perforations | N/A | 1 (2.6) | |
| Patch closure for perforations | N/A | 3 (7.9) | |
| Triangular resection and suture | N/A | 12 (31.6) | |
| Sliding plasty | N/A | 13 (34.2) | |
| Commissural reconstruction | N/A | 4 (10.5) | |
| Neo-chordae | N/A | 5 (13.2) | |
| Annular reconstruction | N/A | 1 (2.6) | |
| Augmentation | N/A | 1 (2.6) | |
| Vegetation resection | N/A | 1 (2.6) | |
| Prosthetic ring | N/A | 34 (89.5) | |
| Associated procedures | |||
| Aortic valve replacement | 1 (1.6) | 0 (0.0) | >0.9999 |
| Tricuspid repair | 18 (28.6) | 17 (44.7) | 0.1311 |
| Postoperative hospital stay (days) | 34.9 ± 23.6 | 16.9 ± 8.4 | <0.0001 |
| In-hospital mortality | 2 (3.2) | 0 (0.0) | 0.5259 |
| Late mortality | 11 (17.5) | 1 (2.6) | 0.0281 |
| Overall mortality | 13 (20.6) | 1 (2.6) | 0.015 |
| Recurrent infective endocarditis | 4 (6.3) | 0 (0.0) | 0.2941 |
| Reintervention | 8 (21.1) | 3 (7.9) | 0.5285 |
| Recurrent mitral regurgitation | 8 (21.1) | 5 (13.2) | >0.9999 |
| Atrial fibrillation in late phase | 21 (33.3) | 2 (5.3) | 0.0011 |
| Late-phase biochemistry profile | |||
| AST (IU/l) | 26.2 ± 11.8 | 20.9 ± 6.9 | 0.0152 |
| LDH (IU/l) | 306.6 ± 88.7 | 218.3 ± 60.2 | <0.0001 |
| Serum potassium (mEq/L) | 4.3 ± 0.6 | 4.3 ± 0.4 | 0.5229 |
Note: Values are mean ± SD or n (%); AST: Aspartate Aminotransferase; CABG: Coronary Artery Bypass Grafting; LDH: Lactate Dehydrogenase.
Study endpoints
During the follow-up period, patients' status was monitored via outpatient clinic visits, by their general practitioners and by telephone interviews. The primary study endpoint was overall mortality, i.e., in-hospital mortality, defined as death occurring within 30 days of the surgery, plus late mortality, defined as death occurring beyond 30 days. Secondary endpoints were reintervention, defined as repeat mitral valve surgery due to MR or recurrent IE and recurrent MR, defined as >Grade 3+ MR. New-onset Atrial Fibrillation (AF) was recorded as an arrhythmic event. Follow-up was continued until the patient died or until termination of the study (December 2022). Mean follow-up time was 61 months (range: 1-333 months).
Statistical analysis
Data are shown as mean ± SD values or as percentages. Between group differences in quantitative variables were analyzed by Mann-Whitney U test and between-group differences in qualitative variables were analyzed by chi-square or Fisher’s exact test. To identify independent predictors of selection of MVR, preoperative characteristics were subjected to logistic regression analysis and Odds Ratios (ORs) (plus 95% Confidence Intervals (95% CIs). Factors for which a P value<0.05 was obtained in univariate analysis were entered into multivariate models and ORs and 95% CIs are shown. Kaplan-Meier curves were drawn for late outcomes of each of the 2 treatments and were analyzed by log-rank test. Risks associated with the primary and secondary endpoints were compared between the 2 groups using univariate analysis for independent variables, variables with a Pvalue<0.20 were included in a Cox proportional hazards model and Hazard Ratios (HRs) and their 95% CIs were calculated. All reported Pvalues were 2-tailed and P<0.05 was considered statistically significant. All analyses were performed with use of GraphPad Prism 9 (GraphPad Software, LLC).
Results
Predictors of selection of MVR
Sixty-three (62.4%) study patients (60 (73.2%) of the 82 patients with active IE and 3 (15.8%) of the patients with healed IE) underwent MVR, whereas 38 (37.6%) study patients (22 (26.8%) of the 82 patients with active IE and 16 (84.2%) of the 19 patients with healed IE) underwent MVRep. Significantly higher percentages of patients in the MVR group (vs. the MVRep group) underwent surgery because of heart failure, uncontrolled sepsis, a systemic embolic event, or mobile vegetation. Results of the logistic regression analyses for predictors of choice of MVR are shown in Table 5. MVR was selected more often for patients of NYHA functional class III or IV, with active IE and with particular indications for surgery (heart failure, uncontrolled sepsis and mobile vegetation) and pathologies (vegetation, large area of leaflet destruction and anterior leaflet prolapse). MVRep was selected more often for patients with chordae rupture and posterior leaflet prolapse. Active IE (OR: 10.6, 95% CI: 2.255 to 71.44; P=0.0060), heart failure as an indication for surgery (OR: 6.43, 95% CI: 1.397 to 36.90; P=0.0233) and a large area of leaflet destruction (OR: 32.3, 95% CI: 3.733 to 885.5; P=0.0080) were shown to be independent predictors of selection of MVR. Age, sex, medical history and causative organisms were not significantly associated with choice of the surgical procedure.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | pValue | OR | 95% CI | Pvalue | |
| Preoperative NYHA functional class III or IV | 3.83 | 1.158-17.40 | 0.045 | - | - | - |
| Stage of endocarditis (active IE) | 14.6 | 4.345-67.00 | <0.0001 | 10.6 | 2.255 -71.44 | 0.006 |
| Indication (heart failure) | 2.89 | 1.138-8.095 | 0.032 | 6.43 | 1.397-36.90 | 0.0233 |
| Indication (uncontrolled sepsis) | 9.62 | 1.793-178.6 | 0.0327 | - | - | - |
| Indication (mobile vegetation) | 2.64 | 1.076-6.998 | 0.0336 | - | - | - |
| Pathology (vegetation) | 3.59 | 1.525-8.743 | 0.0033 | - | - | - |
| Pathology (chordae rupture) | 0.23 | 0.090-0.540 | 0.0011 | - | - | - |
| Pathology (large area of leaflet destruction) | 20.4 | 3.959-373.3 | <0.0001 | 32.3 | 3.733 -885.5 | 0.008 |
| Pathology (posterior leaflet prolapse) | 0.16 | 0.062-0.391 | <0.0001 | - | - | - |
| Pathology (anterior leaflet prolapse) | 2.9 | 1.092-8.676 | 0.0415 | - | - | - |
Note: CI: Confidence Interval; IE: Infective Endocarditis; MRV: Mitral Valve Replacement; MVRep: Mitral Valve Repair; NYHA: New York Heart Association; OR: Odds Ratio.
| Overall mortality | Univariable analysis | Multivariable analysis | ||||
| HR | 95% CI | Pvalue | HR | 95% CI | Pvalue | |
| Age>70 years | 1.08 | 1.031-1.143 | 0.0031 | - | - | - |
| Diabetes mellitus | 6.39 | 0.853- 34.91 | 0.0382 | - | - | - |
| Renal dysfunction | 3.6 | 0.526- 15.69 | 0.1178 | - | - | - |
| Previous cardiac surgery | 3.63 | 0.776- 13.26 | 0.064 | - | - | - |
| Genus Staphylococcus | 3.47 | 0.732- 12.87 | 0.0775 | - | - | - |
| Indication (severe mitral regurgitation) | 4.4 | 1.183- 16.47 | 0.01 | - | - | - |
| Reintervention | Univariable analysis | Multivariable analysis | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Renal dysfunction | 2.99 | 0.448- 12.11 | 0.1691 | - | - | - |
| Genus Staphylococcus | 2.97 | 0.645- 10.37 | 0.1108 | - | - | |
| Indication (uncontrolled sepsis) | 2.97 | 0.776- 9.893 | 0.0832 | - | - | - |
| Pathology (chordae rupture) | 0.13 | 0.007- 0.676 | 0.0511 | - | - | - |
| Associated procedure (tricuspid repair) | 0.16 | 0.009- 0.858 | 0.0847 | - | - | - |
| Recurrent mitral valve regurgitation | Univariable analysis | Multivariable analysis | ||||
| HR | 95% CI | Pvalue | HR | 95% CI | Pvalue | |
| Age>70 years | 2.42 | 0.619- 8.297 | 0.1691 | - | - | - |
| Genus Streptococcus | 0.33 | 0.051- 1.250 | 0.1551 | - | - | - |
| Genus Staphylococcus | 2.47 | 0.551- 8.173 | 0.172 | - | - | - |
| Indication (uncontrolled sepsis) | 2.29 | 0.617- 7.050 | 0.1706 | 8.38 | 1.261 - 61.84 | 0.0277 |
| Indication (severe mitral regurgitation) | 2.6 | 0.676- 8.570 | 0.1289 | - | - | - |
| Pathology (chordae rupture) | 0.24 | 0.036- 0.893 | 0.063 | - | - | - |
| Pathology (extensive leaflet destruction) | 4.02 | 0.784- 73.47 | 0.1827 | - | - | - |
| Associated procedure (tricuspid repair) | 0.31 | 0.048- 1.150 | 0.1258 | - | - | - |
Note: CI: Confidence Interval; HR: Hazard Ratio.
Relation between operative procedure and outcomes
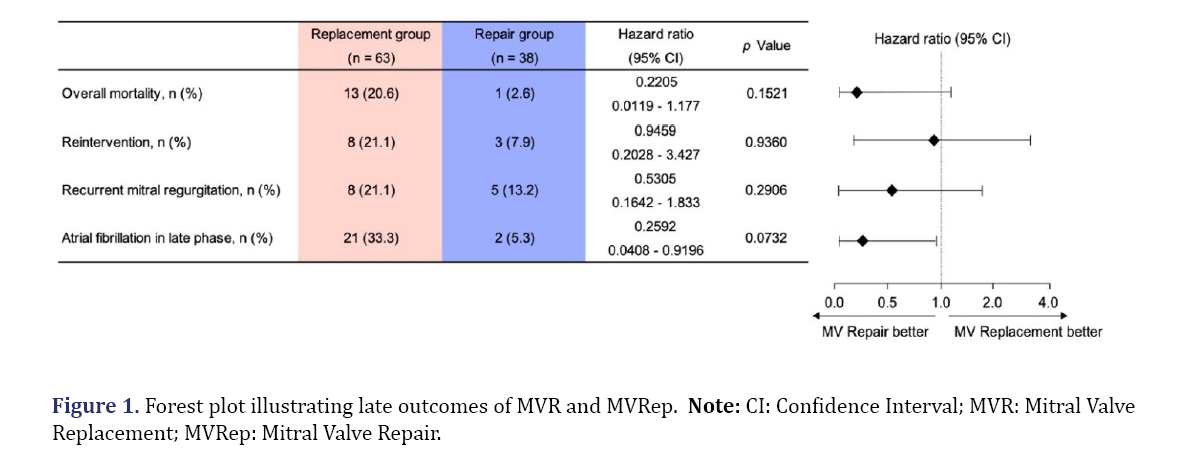
Forest plots of the outcomes of MVR vs. MVRep are given in Figure 1. During the follow-up period, overall mortality was 20.6% in the MVR group and 2.6% in the MVRep group, with the risk of mortality tending to be lower in the MVRep group than in the MVR group (HR: 0.2205, 95% CI: 0.0119 to 1.177; P=0.1521). MVRep tended to be superior to MVR in terms of reintervention (HR: 0.9459, 95% CI: 0.2028 to 3.427; P=0.9360) and of recurrent MR (HR: 0.5305, 95% CI: 0.1642 to 1.833; P=0.2906). The late-period incidence of AF was 33.3% and 5.3% in the MVR group and MVRep group, respectively (HR: 0.2592, 95% CI: 0.0408 to 0. 9196; P=0.0732) (Figure 2).
Mortality
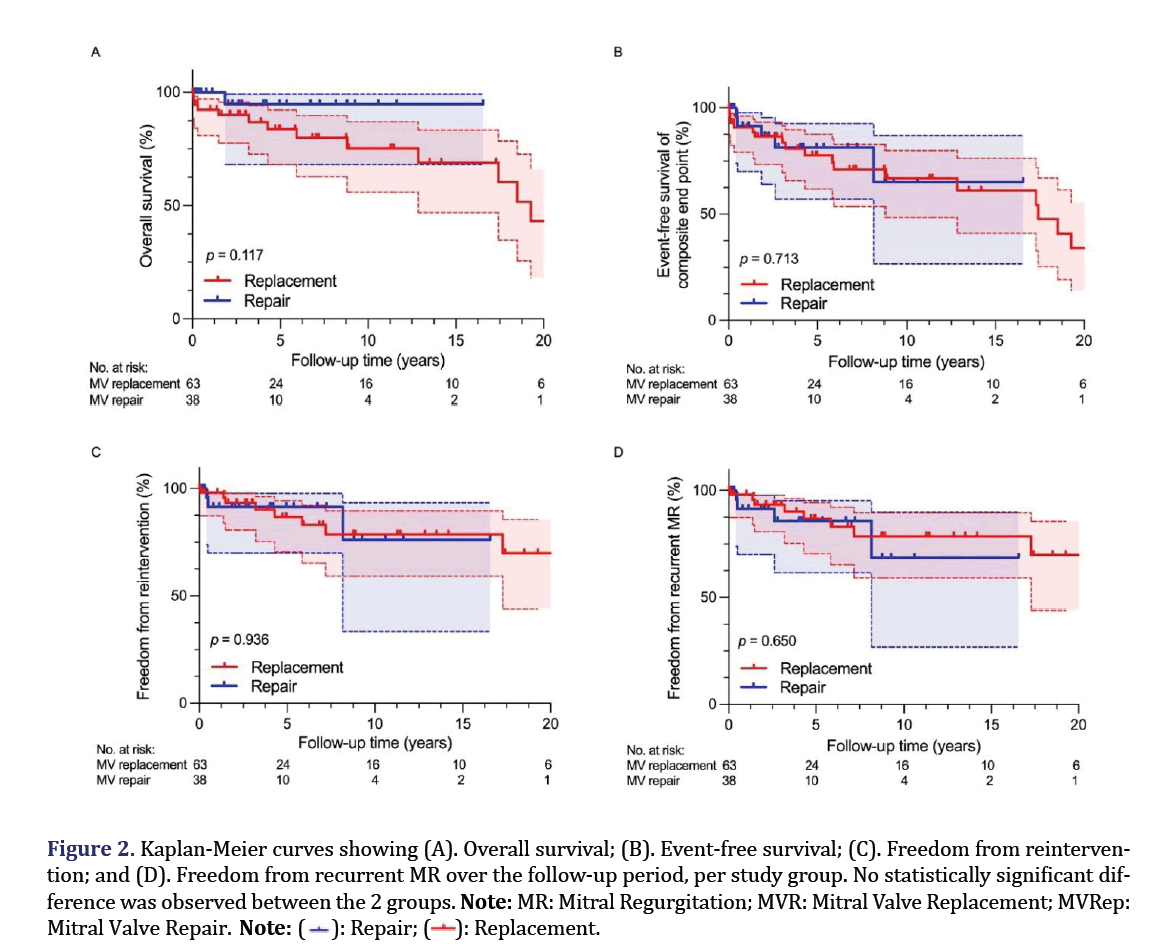
There were 2 in-hospital deaths (2.0%) (2 (3.2%) in the MVR group and 0 (0.0%) in the MVRep group; P=0.5259). One of the 2 deaths was of an 86-year-old woman with multiple cerebral embolisms associated with the IE. After MVR, bioprosthetic valve IE developed, leading to perivalvular regurgitation and left ventricular rupture and the woman died 36 days after the surgery. The other was of a 79-year-old woman who died of multiple organ failure 29 days after repeat MVR for recurrent IE. Death after 30 days occurred in 12 cases (11.9%) (11 cases (17.5%) in the MVR group and 1 case (2.6%) in the MVRep group, P=0.0281). Deaths in the MVR group were due to intracranial hemorrhage (n=2), stroke (n=1), congestive heart failure (n=5), or fatal ventricular arrhythmia (n=1) or were sudden and of unknown cause (n=1) or not of cardiovascular origin (n=1). Death in the MVRep group was due to congestive heart failure. Kaplan-Meier curves for overall survival and event-free survival are shown in Figures 2A and 2B. Five-year survival was 83.8% (95% CI: 68.12 to 92.14) in the MVR group and 94.7% (95% CI: 66.12 to 99.24) in the MVRep group (P=0.1789). Ten-year survival was 75.2% (95% CI: 55.96 to 87.00) in the MVR group and 94.7% (95% CI: 68.12 to 99.24) in the MVRep group (P=0.1232). Overall survival was 43.1% (95% CI: 18.02 to 66.16) in the MVR group and 94.7% (95% CI: 68.12 to 99.24) in the MVRep group (P=0.1168). Five-year event-free survival was 77.7% (95% CI: 61.87 to 87.60) in the MVR group and 86.7% (95% CI: 64.00 to 95.51) in the MVRep group (P=0.3700). Ten-year event-free survival was 66.8% (95% CI: 48.33 to 79.92) in the MVR group and 72.2% (95% CI: 33.65 to 90.73) in the MVRep group (P=0.4330). Overall event-free survival was 34.0% (95% CI: 13.85 to 55.52) in the MVR group and 36.1% (95% CI: 1.514 to 78.06) in the MVRep group (P=0.7140). Survival and event-free survival did not differ significantly between the 2 groups at each observation period.
Figure 2. Kaplan-Meier curves showing (A). Overall survival; (B). Event-free survival; (C). Freedom from reintervention; and (D). Freedom from recurrent MR over the follow-up period, per study group. No statistically significant difference was observed between the 2 groups.
Note: MR: Mitral Regurgitation; MVR: Mitral Valve Replacement; MVRep: Mitral Valve Repair. 
Preoperative clinical variables and their relation to mortality were examined and are shown in Table 6. On univariate analysis, age >70 years, diabetes mellitus and severe MR as a surgical indication were found to be significantly associated with mortality. Renal dysfunction, previous cardiac surgery and genus Staphylococcus as causative organisms were found to affect mortality, but not significantly. However, after adjustment for these variables in Cox proportional hazards analysis, no significant independent predictors of mortality were identified.
Recurrent MR
Thirteen patients (12.9%) experienced recurrent MR (8 (21.1%) in the MVR group and 5 (13.2%) in the MVRep group), with no significant difference between the 2 groups (P>0.9999). The 8 patients in the MVR group were the same as those requiring reintervention. Three of the 5 patients in the MVRep group were the same as those requiring reintervention and of the remaining 2, 1 was a patient who had undergone triangular resection and suture and the other was a patient who had undergone sliding plasty. Kaplan-Meier recurrent MR-free survival curves are shown for the 2 groups in Figures 2C and 2D. Freedom from recurrent MR over the entire follow-up period was 69.9% (95% CI: 43.95 to 85.55) in the MVR group and 38.1% (95% CI: 1.388 to 80.45) in the MVRep group (P=0.6214). Preoperative clinical variables are shown in relation to recurrent MR in Table 6. On univariate analysis, age >70, genus Streptococcus as the causative organism, genus Staphylococcus as the causative organisms, uncontrolled sepsis, severe MR, chordae rupture, a large area of leaflet destruction and tricuspid repair as an associated procedure were shown to be factors affecting recurrent MR. After adjustment for these variables in Cox proportional hazards analysis, uncontrolled sepsis (HR: 8.38, 95% CI: 1.261 to 61.84; P=0.0277) was identified as a significant independent predictor of recurrent MR.
Echocardiography
Echocardiographic outcomes are shown separately for the MVR (mechanical and biological valves) and MVRep groups in Table 7. Follow-up TTE was performed in all patients. Preoperatively, 74 patients (73.3%) had severe MR. There was no significant difference in preoperative LAD, LVDd, or LVDs between the MVR (mechanical and biological valves) and MVRep groups, but the Tricuspid Regurgitation Pressure Gradient (TR-PG) was significantly higher in the MVR group than in the MVRep group. This is reflective of the fact that more patients in the MVR group than in the MVRep group had NYHA III or IV heart failure. In both the MVR (mechanical and biological valves) and MVRep groups, LVDd and LVDs were significantly reduced due to reverse remodeling in the late postoperative period. Notably, only in the MVRep group, the peak mitral inflow velocity, maximum trans-mitral pressure gradient and mean trans-mitral pressure gradient were significantly decreased in the late period compared values in the preoperative period. Although the data must be interpreted by taking into account that fewer patients in the MVRep group than in the MVR group were at risk, valve performance (i.e., reduction in left atrial load) was better after MVRep than after MVR.
|
|
Preoperative period | Early postoperative period | Late postoperative period | Pvalue | ||
|---|---|---|---|---|---|---|
| Pre vs. Early | Pre vs. Late | Early vs. Late | ||||
| Mechanical valve replacement | ||||||
| LAD (mm) | 47.1 ± 10.0 | 44.0 ± 9.6 | 45.1 ± 12.2 | 0.0873 | 0.3962 | 0.3962 |
| LVDd (mm) | 53.9 ± 6.9 | 48.9 ± 6.7 | 46.7 ± 7.8 | 0.0001 | <0.0001 | 0.058 |
| LVDs (mm) | 33.9 ± 6.0 | 34.7 ± 6.3 | 30.0 ± 6.5 | 0.4253 | 0.0009 | 0.0007 |
| LVEF (%) | 66.1 ± 8.7 | 56.0 ± 10.1 | 65.3 ± 8.6 | 0.0002 | 0.699 | 0.0001 |
| TR-PG (mmHg) | 33.3 ± 16.8 | 21.1 ± 5.8 | 22.5 ± 9.1 | 0.0017 | 0.0102 | 0.3098 |
| E/e' | 21.7 ± 10.6 | 27.2 ± 9.5 | 31.2 ± 9.6 | 0.2034 | 0.2034 | 0.2408 |
| MV peak velocity (m/s) | 1.6 ± 0.3 | 1.8 ± 0.3 | 1.7 ± 0.4 | 0.3237 | 0.6491 | 0.1269 |
| MV max PG (mmHg) | 11.5 ± 5.0 | 13.1 ± 5.4 | 11.9 ± 5.4 | 0.5944 | 0.6779 | 0.3962 |
| MV mean PG (mmHg) | 4.3 ± 2.7 | 5.4 ± 2.0 | 4.2 ± 1.5 | 0.4305 | 0.9594 | 0.0043 |
| Bioprosthetic replacement valve | ||||||
| LAD (mm) | 52.7 ± 9.3 | 46.2 ± 7.8 | 47.3 ± 10.3 | 0.0267 | 0.1023 | 0.5884 |
| LVDd (mm) | 53.1 ± 7.0 | 45.3 ± 7.8 | 43.6 ± 6.2 | 0.01 | 0.0003 | 0.4375 |
| LVDs (mm) | 34.0 ± 5.7 | 31.7 ± 7.2 | 29.6 ± 5.1 | 0.2418 | 0.0011 | 0.2418 |
| LVEF (%) | 66.7 ± 4.5 | 57.0 ± 12.6 | 60.4 ± 9.0 | 0.0525 | 0.0669 | 0.2368 |
| TR-PG (mmHg) | 38.9 ± 15.3 | 25.0 ± 6.7 | 25.6 ± 8.7 | 0.0102 | 0.0153 | 0.7788 |
| E/e' | 31.6 ± 17.6 | 32.9 ± 6.4 | 28.0 ± 7.7 | 0.7673 | 0.746 | 0.0038 |
| MV peak velocity (m/s) | 2.5 ± 1.0 | 1.9 ± 0.5 | 1.6 ± 0.4 | 0.3108 | 0.251 | 0.0602 |
| MV max PG (mmHg) | 22.8 ± 20.6 | 15.7 ± 9.3 | 10.8 ± 4.7 | 0.4548 | 0.3657 | 0.0812 |
| MV mean PG (mmHg) | 5.3 ± 2.7 | 6.3 ± 4.9 | 4.4 ± 1.7 | 0.7311 | 0.4898 | 0.256 |
| Valve repair | ||||||
| LAD (mm) | 47.9 ± 10.1 | 40.8 ± 7.8 | 44.0 ± 10.1 | 0.0001 | 0.0677 | 0.0677 |
| LVDd (mm) | 55.3 ± 6.9 | 48.2 ± 7.2 | 46.6 ± 5.2 | <0.0001 | <0.0001 | 0.1479 |
| LVDs (mm) | 34.9 ± 6.2 | 33.4 ± 6.5 | 30.0 ± 5.1 | 0.0611 | 0.0002 | 0.0089 |
| LVEF (%) | 65.7 ± 8.0 | 58.0 ± 11.1 | 64.9 ± 8.5 | 0.001 | 0.6466 | 0.0081 |
| TR-PG (mmHg) | 23.1 ± 8.3*# | 18.1 ± 7.5# | 18.3 ± 7.3# | 0.0149 | 0.0884 | 0.8458 |
| E/e' | 18.1 ± 8.0# | 23.0 ± 9.4# | 28.3 ± 10.1 | 0.0486 | 0.0035 | 0.0486 |
| MV peak velocity (m/s) | 2.3 ± 0.1 | 1.3 ± 0.3*# | 1.4 ± 0.3* | 0.2233 | <0.0001 | 0.2233 |
| MV max PG (mmHg) | 16.8 ± 8.2 | 6.8 ± 3.3*# | 8.2 ± 2.9* | 0.1338 | <0.0001 | 0.1644 |
| MV mean PG (mmHg) | 6.3 ± 3.7 | 2.8 ± 1.5*# | 3.0 ± 0.9*# | 0.0447 | <0.0001 | 0.3611 |
Note: Values are mean ± SD. *P<0.05 vs. mechanical valve replacement; #P<0.05 vs. Bioprosthetic valve replacement. LAD: Left Atrial Dimension; LVDd: Left Ventricular End-Diastolic Dimension; LVDs: Left Ventricular End-Systolic Dimension; LVEDV: Left Ventricular End-Diastolic Volume; LVEF: Left Ventricular Ejection Fraction; LVESV: Left Ventricular End-Systolic Volume; MV: Mitral Valve; SV: Systolic Volume; TR-PG: Tricuspid Regurgitation Pressure Gradient.
Discussion
Our study comparing outcomes of MVR and MVRep points to the benefits of MVRep with respect to survival, recurrence of MR and incidence of AF when performed in patients with MR caused by either active or healed IE. To our knowledge, this is the first study to examine changes in echocardiographic variables from the preoperative to early and late postoperative periods in patients treated for mitral valve IE and to address the relation between cardiac functional outcome and the incidence of postoperative AF.
In principle, infected tissue should be completely excised without consideration for the effect of the procedure on the subsequent MVRep. If valve destruction is extensive, MVR is inevitable, but early surgery prevents the progression of tissue destruction and allows for durable repair [13]. Although concerns have been raised regarding the durability of complicated MVRep for inflamed tissue in cases of active IE, Dreyfus et al. performed MVRep in patients with active IE and reported good results [1]. MVRep was selected for 22 (26.8%) of our 82 study patients with active IE and 16 (84.2%) of our 19 patients with healed IE. MVRep was selected more frequently for patients included in our study than for those included in a retrospective database study conducted by Toyoda et al. [8]. Their study covered 1970 patients in California and New York who underwent mitral valve surgery for active IE and MVRep was selected in 10.7%-19% of cases.
Selection of the procedure is known to be influenced by the apparent pathology and Muehrcke et al. reported that patients with vegetations on the anterior or posterior leaflets and a history of MVRep were more likely than others to require MVR [14]. Among our study patients, MVR was selected significantly more frequently when a large area of leaflet destruction and/or anterior leaflet prolapse was present. Furthermore, preoperative severe heart failure, uncontrolled sepsis, occurrence of a systemic embolic event and mobile vegetation were more prevalent in our MVR group than in our MVRep group, suggesting that preoperative hemodynamic instability and the need for emergency surgery led to the choice of immediate MVR rather than complex MVRep to avoid prolonged ischemia time.
Recently, favorable outcomes in terms of survival and durability of MVRep performed for active IE have been reported. Lung et al. evaluated the feasibility and outcomes of repair procedures for both active and healed IE and showed MVRep to be feasible in 78% of patients with active IE and 83% of patients with healed IE; survival rates were excellent [15]. Previous reports have also shown 5-year survival rates of 85% to 93% for patients undergoing MVRep for IE [16,17]. Five-year survival in our MVR group and our MVRep group was 83.8% and 94.7%, respectively, consistent with rates previously reported. Muehrcke et al. and Sternik et al. reported better early and late mortality and event-free survival in patients who underwent MVRep for active IE than for those who underwent MVR [14,18]. Ruttmann et al. also reported that MVRep (vs. MVR) in patients with active IE significantly improved survival [19]. Although we were unable to show a statistical advantage, we did document a trend toward improved overall survival in the MVRep group compared to that in the MVR group (Figure 2A). Thus, we believe MVRep can be considered a reliable option after thorough evaluation of valve damage. We also did not identify independent predictors of postoperative mortality, but previous reported studies have identified preoperative septic shock, stroke and IE caused by Staphylococcus aureus as independent predictors of mortality [20,21]. Our univariate analysis also showed an association between Staphylococcus aureus infection and mortality, reintervention and recurrent MR, as noted above.
MVRep remains an attractive procedure because, in comparison to MVR, it better preserves left ventricular function and reduces the incidence of valve-related events [22]. Reintervention was required in only 7.9% of patients in our MVRep group (as opposed to 21.1% in our MVR group) and freedom from reintervention over the entire follow-up period was 76.2% in our MVRep group (as opposed to 69.9% in our MVR group). MVR was not shown in our study to be statistically superior to MVRep in preventing reintervention, but the reintervention rate for MVRep was slightly lower than that for MVR. Previous reports have shown superiority of MVRep in preventing reintervention (7.9% to 8.7% after MVR vs. 2.6% to 7.9% after MVRep) [6,23]. Although our study did not identify independent predictors of reintervention, presence of a paravalvular abscess and calcification and rheumatic disease have been shown previously to be predictors [2].
Thirteen (12.9%) of our study patients experienced recurrent MR, with freedom from MR recurrence during the entire follow-up period being 69.9% in the MVR group and 76.2% in the MVRep group. This outcome was comparable to the 73% freedom from MR recurrence at 12 years following performance of MVRep for degenerative disease reported by David et al. [24]. Uncontrolled sepsis was identified as an independent predictor of MR recurrence in our multivariable analysis, suggesting that radical resection of infected tissue is important to prevent recurrence of MR.
Patients with IE often present with congestive heart failure. Progressive left ventricular dilatation is associated with poor long-term prognosis, whereas reverse remodeling is associated with good prognosis [25]. TTE performed consecutively in our study patients confirmed reverse remodeling of the left ventricle in both the MVR group and MVRep group. The hemodynamic superiority of MVRep was described by Zehr as follows. The recreation of the anatomy by mitral valve repair allows for nonturbulent inflow into the left ventricle and unimpeded laminar flow through the left ventricular outflow tract. The left ventricular geometrical dimensions are maintained with the chordal preservation associated with the repair. This translates to normalizing flow and contractility. In replaced patients, turbulent flow patterns likely place the patient at incremental risk for recurrent endocarditis and result in increased transvalvular gradients both across the mitral valve and the left ventricular outflow tract.
The lower incidence of AF in our MVRep group may be fundamentally due to the reduced left atrial load resulting from the decreased trans-mitral blood flow velocity and pressure gradient compared to those in the MVR group. The benefits of MVRep with respect to left ventricular function are well established when MVRep is performed for degenerative disease [3].
Study limitations
Limitations of the study include, first, its design as a retrospective, nonrandomized, single-center observational study. Second, the sample size was small and the mean follow-up period was short. With a larger sample size and longer follow-up period, results might differ. Third, MVR was often performed in patients with extensive valve destruction that did not allow for MVRep. Poor postoperative outcomes can be expected in such patients. Fourth, surgical techniques and approaches, which have improved over the past 30 years, may have influenced the study results. Further research is needed on the relative benefits of MVRep vs. MVR and on various issues such as long-term clinical outcomes in cases of active vs. healed IE, reverse remodeling of the ventricle and association between left atrial load and AF.
Conclusion
MVRep is an attractive surgical option for patients with mitral valve IE due to its favorable long-term prognosis, reduction of MR recurrence, improved cardiac function and low incidence of AF and our study too showed that MVRep for IE preserves left ventricular function and reduces the incidence of AF by significantly decreasing left atrial load.
Declarations
Acknowledgments
We thank Ms. Wendy Alexander-Adams for her assistance in reporting our findings in English.
Data availability statement
The patients data used to support the findings of this study are restricted by the Institutional Review Board of Jichi Medical University in order to protect patient privacy. Data are available from Manabu Shiraishi, (E-mail: manabu@omiya.jichi.ac.jp ) for researchers who meet the criteria for access to confidential data.
Funding statement
No special funding outside of departmental resources.
Conflict of interest disclosure
No conflicts of interest to disclose.
Institutional review board approval
Approval no. S22-102.
Patient consent statement
Patients waived informed consent by the opt-out method.
Permission to reproduce material from other sources
Not applicable.
Clinical trial registration
Not applicable for this study.
Author contributions
Study design, analysis and interpretation: Manabu Shiraishi.
Drafting of the manuscript: Manabu Shiraishi.
Critical revision of the manuscript: Manabu Shiraishi, Naoyuki Kimura and Atsushi Yamaguchi.
Approval of the submitted and final version: Manabu Shiraishi, Shigeto Tokunaga, Kengo Teshima, Hiroki Arai, Naoyuki Kimura and Atsushi Yamaguchi.
References
- Dreyfus G, Serraf A, Jebara VA, Deloche A, Chauvaud S, Couetil JP, et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990;49(5):706-713.
[Crossref] [Google Scholar] [Pubmed]
- de Kerchove L, Price J, Tamer S, Glineur D, Momeni M, Noirhomme P, et al. Extending the scope of mitral valve repair in active endocarditis. J Thorac Cardiovasc Surg 2012;143(4):S91-S95.
[Crossref] [Google Scholar] [Pubmed]
- Galloway AC, Colvin SB, Baumann FG, Esposito R, Vohra R, Harty S, et al. Long-term results of mitral valve reconstruction with carpentier techniques in 148 patients with mitral insufficiency. Circulation 1988;78(3 Pt 2):I97-105.
[Google Scholar] [Pubmed]
- Cosgrove DM, Chavez AM, Lytle BW, Gill CC, Stewart RW, Taylor PC, et al. Results of mitral valve reconstruction. Circulation 1986;74(3 Pt 2):I82-87.
[Google Scholar] [Pubmed]
- Deloche A, Jebara VA, Relland JY, Chauvaud S, Fabiani JN, Perier P, et al. Valve repair with carpentier techniques: The second decade. J Thorac Cardiovasc Surg 1990;99(6):990-1002.
[Crossref] [Google Scholar] [Pubmed]
- Feringa HH, Shaw LJ, Poldermans D, Hoeks S, van der Wall EE, Dion RA, et al. Mitral valve repair and replacement in endocarditis: A systematic review of literature. Ann Thorac Surg 2007;83(2):564-570.
[Crossref] [Google Scholar] [Pubmed]
- Lee HA, Cheng YT, Wu VC, Chou AH, Chu PH, Tsai FC, et al. Nationwide cohort study of mitral valve repair versus replacement for infective endocarditis. J Thorac Cardiovasc Surg 2018;156(4):1473-1483.
[Crossref] [Google Scholar] [Pubmed]
- Toyoda N, Itagaki S, Egorova NN, Tannous H, Anyanwu AC, El-Eshmawi A, et al. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg 2017;154(6):1906-1912.
[Crossref] [Google Scholar] [Pubmed]
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30(4):633-638.
[Crossref] [Google Scholar] [Pubmed]
- Shiraishi M, Kimura N, Yamaguchi A. Early cardiac contractility outcome of reoperative coronary artery bypass grafting using right gastroepiploic artery. J Card Surg 2021;36(11):4103-4110.
[Crossref] [Google Scholar] [Pubmed]
- Thomas JD. How leaky is that mitral valve? Simplified doppler methods to measure regurgitant orifice area. Circulation 1997;95(3):548-550.
[Crossref] [Google Scholar] [Pubmed]
- Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, Lessana A, et al. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg 1980;79(3):338-348.
[Crossref] [Google Scholar] [Pubmed]
- Defauw RJ, Tomšič A, van Brakel TJ, Marsan NA, Klautz RJ, Palmen M, et al. A structured approach to native mitral valve infective endocarditis: Is repair better than replacement?. Eur J Cardiothorac Surg 2020;58(3):544-550.
[Crossref] [Google Scholar] [Pubmed]
- Muehrcke DD, Cosgrove DM, Lytle BW, Taylor PC, Burgar AM, Durnwald CP, et al. Is there an advantage to repairing infected mitral valves?. Ann Thorac Surg 1997;63(6):1718-1724.
[Crossref] [Google Scholar] [Pubmed]
- Iung B, Rousseau-Paziaud J, Cormier B, Garbarz E, Fondard O, Brochet E, et al. Contemporary results of mitral valve repair for infective endocarditis. J Am Coll Cardiol 2004;43(3):386-392.
[Crossref] [Google Scholar] [Pubmed]
- Zegdi R, Debièche M, Latrémouille C, Lebied D, Chardigny C, Grinda JM, et al. Long-term results of mitral valve repair in active endocarditis. Circulation 2005;111(19):2532-2536.
[Crossref] [Google Scholar] [Pubmed]
- Mihaljevic T, Paul S, Leacche M, Rawn JD, Aranki S, O'Gara PT, et al. Tailored surgical therapy for acute native mitral valve endocarditis. J Heart Valve Dis 2004;13(2):210-216.
[Google Scholar] [Pubmed]
- Sternik L, Zehr KJ, Orszulak TA, Mullany CJ, Daly RC, Schaff HV, et al. The advantage of repair of mitral valve in acute endocarditis. J Heart Valve Dis 2002;11(1):91-97.
[Google Scholar] [Pubmed]
- Ruttmann E, Legit C, Poelzl G, Mueller S, Chevtchik O, Cottogni M, et al. Mitral valve repair provides improved outcome over replacement in active infective endocarditis. J Thorac Cardiovasc Surg 2005;130(3):765-771.
[Crossref] [Google Scholar] [Pubmed]
- Musci M, Hübler M, Amiri A, Stein J, Kosky S, Meyer R, et al. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience. Eur J Cardiothorac Surg 2010;38(5):528-538.
[Crossref] [Google Scholar] [Pubmed]
- Oliveira JL, Santos MA, Arnoni RT, Ramos A, Togna DD, Ghorayeb SK, et al. Mortality predictors in the surgical treatment of active infective endocarditis. Braz J Cardiovasc Surg 2018;33:32-39.
[Crossref] [Google Scholar] [Pubmed]
- Yamaguchi H, Eishi K, Yamachika S, Hisata Y, Tanigawa K, Izumi K, et al. Mitral valve repair in patients with infective endocarditis. Circ J 2006;70(2):179-183.
[Crossref] [Google Scholar] [Pubmed]
- Tepsuwan T, Rimsukcharoenchai C, Tantraworasin A, Taksaudom N, Woragidpoonpol S, Chuaratanaphong S, et al. Comparison between mitral valve repair and replacement in active infective endocarditis. Gen Thorac Cardiovasc Surg 2019;67(12):1030-1037.
[Crossref] [Google Scholar] [Pubmed]
- David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130(5):1242-1249.
[Crossref] [Google Scholar] [Pubmed]
- Udelson JE, Konstam MA. Relation between left ventricular remodeling and clinical outcomes in heart failure patients with left ventricular systolic dysfunction. J Card Fail 2002;8(6):S465-S4671.
[Crossref] [Google Scholar] [Pubmed]