Case Series - Archives of Clinical and Experimental Surgery (2023)
Outcomes Following Management of Congenital and Acquired Diaphragmatic Hernia in a Tertiary Care Centre: A Case Series
Saikalyan A Guptha*, Ravikumar H, Sidduraj C Sajjan, Tinnu George, Karthik HS and Harshita RSaikalyan A Guptha, Department of General Surgery, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, India, Tel: +919663313222, Email: drsaikalyanguptha@gmail.com
Received: 16-Dec-2022, Manuscript No. EJMACES-23-83796; Editor assigned: 19-Dec-2022, Pre QC No. EJMACES-23-83796 (PQ); Reviewed: 04-Jan-2023, QC No. EJMACES-23-83796; Revised: 10-Jan-2023, Manuscript No. EJMACES-23-83796 (R); Published: 18-Jan-2023
Abstract
Introduction: Congenital Diaphragmatic Hernia (CDH) is a congenital abnormality, frequently seen in children. With the advent of prenatal ultrasound, it is diagnosed prenatally and corrected in the pediatric age. Rarely adults present to the emergency room with congenital diaphragmatic hernia. Traumatic diaphragmatic hernia is also not unheard of and seen in a wide variety of blunt trauma injuries in road traffic accidents. Congenital diaphragmatic hernia occurs in 1 in 2,200 to 5000 live births. Early diagnosis and prompt surgical intervention results in positive outcomes. This article will provide our experience with the diaphragmatic hernia and the outcomes.
Methods: From 2018 to 2021, five patients of diaphragmatic hernia were admitted to the Vydehi Hospital. Patients underwent successful surgery to treat the diaphragmatic hernia. They were operated by the same team of surgeons. The presentation of each patient, difficulties in each case, the treatment provided and the outcomes following the surgery is described in this article, with facts reviewed from the hospital medical records.
Results: Case 1 presented with a Bochdalek hernia with right kidney as content. Case 2 presented with gastric volvulus and splenic flexure of colon as content. Case 3 presented with traumatic diaphragamatic hernia with stomach as content of hernia. Case 4 presented with adult congenital diaphragmatic hernia with small bowel and part of large bowel as content. Case 5 presented with Bochdalek hernia with transverse colon, spleen, fundus of the stomach, omentum, and part of small bowel as the content.
Conclusion: Adult Congenital Diaphragmatic Hernia is a very unusual presentation. Prenatal ultrasound allows for early diagnosis. Pulmonary hypertension and pulmonary hypoplasia if present reduces the survival chances. Patients who present in their adulthood with congenital diaphragmatic hernia need surgical correction.
Keywords
Diaphragmatic hernia; Diaphragmatic rupture; Mesh; Emergency surgery; Laparotomy
Introduction
Diaphragmatic Hernia (DH) is a defect of the diaphragm which allows the passage of an organ, or part of it, into the thoracic cavity [1]. Diaphragmatic hernia can be congenital or acquired. Congenital Diaphragmatic Hernia (CDH) incidence is 1 in 2,000 to 5000 live births [2]. High rate of in utero fetal demise, still birth and early neonatal death prior to transfer to a tertiary referral center, true incidence is difficult to capture [3]. With the advent of prenatal ultrasound, two thirds of the CDH is detected by second trimester [4]. Prenatal ultrasound is successful in making the diagnosis of CDH as early as 15 weeks of gestation.
In 1679, first CDH was recorded by Lazarus Riverius (1589–1655), an incidental postmortem finding in a 24 year old man [5]. First pediatric account of CDH in English literature was recorded by Sir Charles holt in 1701 in “Philosophical Transactions of Royal Society of London” which classically describes the clinical and postmortem findings of an infant of CDH [5]. In 1761 Giovanini Battista Morgagni (1682–1771) described various types of diaphragmatic hernias, including the anterior diaphragmatic hernia which bears his name [5]. René Théophile Hyacinthe Laënnec (1781–1826) invented stethoscope, the technique of auscultation in the diagnosis of diaphragmatic hernia and first proposed possibility of surgical reduction via an abdominal approach [5]. Sir Astley cooper (1768–1841) gave detailed clinical and anatomical description of diaphragmatic hernia and the anatomical cause for diaphragmatic hernia [5]. In 1847, Henry I Bowditch was the first to make a bedside diagnosis of diaphragmatic hernia and emphasize the clinical criteria for making the diagnosis [5]. In 1848, Victor Alexander Bochdalek, a professor of anatomy at prague, described CDH occurring through both right and left posterolateral diaphragmatic defects. In 1888, first surgical attempt at repair of CDH. Nauman of Swe-den performed a celiotomy on a 19-year-old patient with CDH, who died of sepsis [5]. Nauman proposed a two-cavity approach. In 1889, Dr. J. O’Dwyer of New York, performed the first abdominal approach for repair of CDH [5]. In 1905, first successful repair of CDH reported in the German literature by Heidenhain of the municipal Hospital of Worms, Germany. In 1929, Bettman and Hess reported successful repair of CDH in an infant of 3½ month old.
Before 1940, Gross and Ladd reported 9 out of 16 survivors of CDH with immediate surgical repair. As experts believed the inciting factor for mortality was mediastinal compression from the herniated viscera. Surgical repair became the main stay of therapy. But the survival was at best around 50%. In the mid-1980s Dr. Stolar recognized the impact of Pulmonary Hypoplasia (PH) associated with CDH and introduced the idea of permissive hypercapnia resulted in a paradigm shift. Current expected survival for CDH may be as high as 70% to 80 % [3].
The Pleuroperitoneal cavities become separated by the developing membrane during 8 to 10 weeks of gestation. If the pleuroperitoneal canal persists, leads to a posterolateral CDH defect known as Bochdalek hernia. CDH with an anteromedial defect known as Morgagni hernia. Abdominal contents herniate through the defect, compressing the ipsilateral lung [6]. The lungs have pulmonary hypoplasia, ipsilateral lung affected more severely. Pulmonary vasculature is significantly affected by the increased thickness of arteriolar smooth muscle and extremely sensitive to local and systemic vasoactive factors [2].
CDH in adults is considerably rare as the diagnosis is made prenatally or in infancy. There is a subset of patients who present in their late teens and early 20s with worsening symptoms of breathlessness and abdominal pain. A keen clinical examination raises the suspicion of CDH. Chest X-ray is usually diagnostic [6].
Patient and methods
Study setting: The study was conducted in department of surgery, Vydehi Institute of medical sciences and research center, Bengaluru.
Study design: Retrospective Case series analysis. From 2018 to 2021, five patients of diaphragmatic hernia were admitted to the Vydehi Hospital. Patient underwent successful surgery to treat the diaphragmatic hernia. Data was obtained from the case records and analysed.
Case Presentation
Case 1
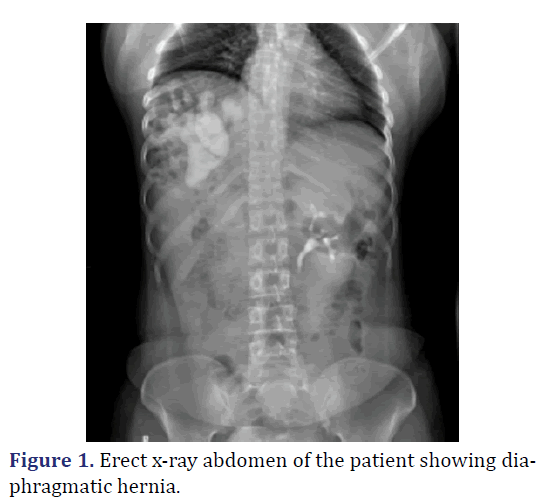
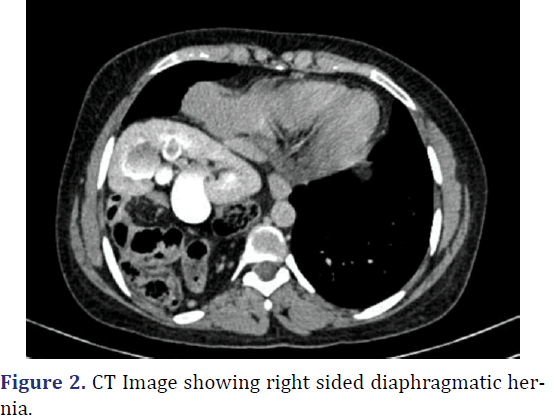
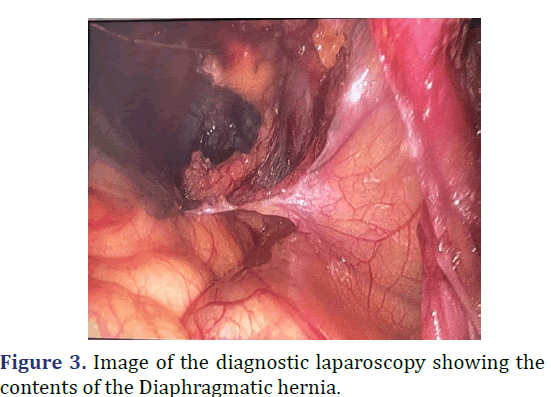
A 26-year-old female was admitted with complaints abdominal pain in the last 3 months. Patient history revealed pain abdomen associated with burning micturition. Physical examination revealed tenderness in the right iliac and lumbar regions. Gurgling sounds (Bowel sounds) heard in the right sided lower auscultatory areas of the chest. Chest radiograph revealed elevated right dome of the diaphragm with interposition of bowel loops between the diaphragm and liver. Ultrasonography of abdomen revealed Ectopic right kidney in the right intrathoracic region with malformation of the renal pelvis causing narrowing of Pelvi-ureteric junction. Multi-disciplinary approach to surgery with general surgery and urological surgery teams. Diagnostic laparoscopy revealed right sided diaphragmatic defect with transverse colon with hepatic flexure, malrotated ectopic right kidney within the thoracic cavity. Laparoscopic reduction of hernial contents done. Procedure converted to open. Using Kocher’s incision, excision of the pleuroperitoneal sac, with anatomical repair of the diaphragm with mesh reinforcement and nephropexy done. Post-operative period was uneventful. Patient was discharged on 7th post op day (Figures 1-3).
Case 2
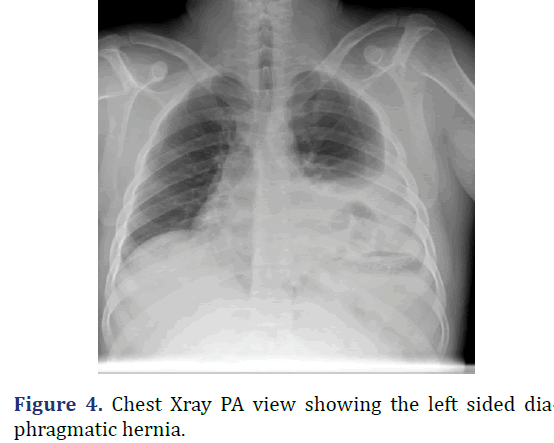
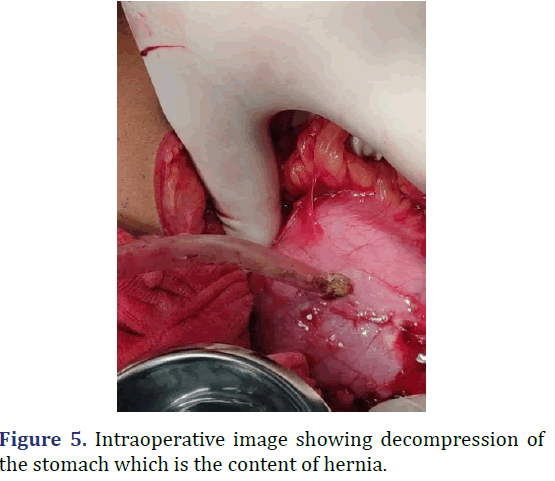
A 30 year old male admitted with abdominal pain and vomiting since 2 days. Patient history revealed upper abdomen pain, sudden onset and severe, associated with vomiting. Physical examination revealed distended abdomen with guarding and tenderness in the hypochondriac and epigastric regions, with reduced breath sounds on the left side of the chest. Chest radiograph revealed hyper-elevated left dome of the diaphragm with gastric air shadow in the chest with reduced left sided lung shadow. CECT abdomen revealed large defect in the left hemidiaphragm 10.5 cm × 9.9 cm with herniation of the pylorus of the stomach, splenic flexure of the colon and omentum into the left hemithorax suggestive of diaphragmatic hernia. Emergency laparotomy was performed. Stomach and splenic flexure of the large bowel as content of the hernia noted, intact diaphragm noted. Derotation of the stomach to its anatomical position done, colon and omentum reduced to the abdominal cavity, stomach secured to the diaphragm, gastropexy and a feeding jejunostomy done. On the 9th post-operative day patient developed burst abdomen for which tension suturing was done. Following which patient recovered satisfactorily. Patient was discharged on 12th post-operative day (Figures 4 and 5).
Case 3
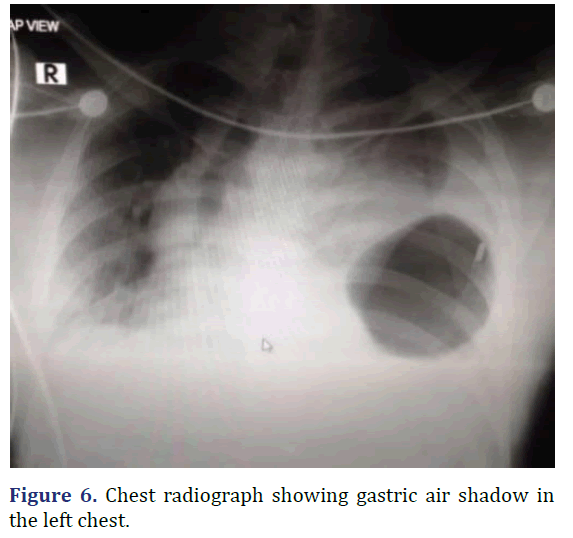
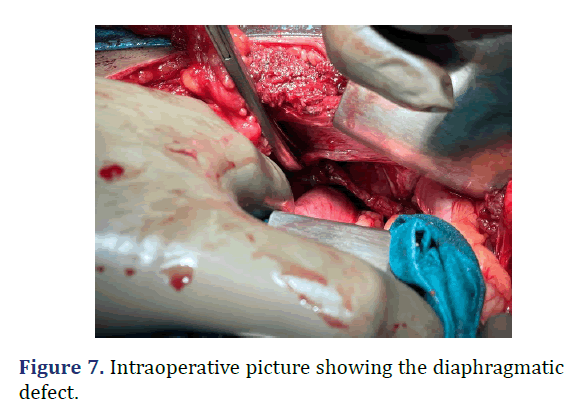
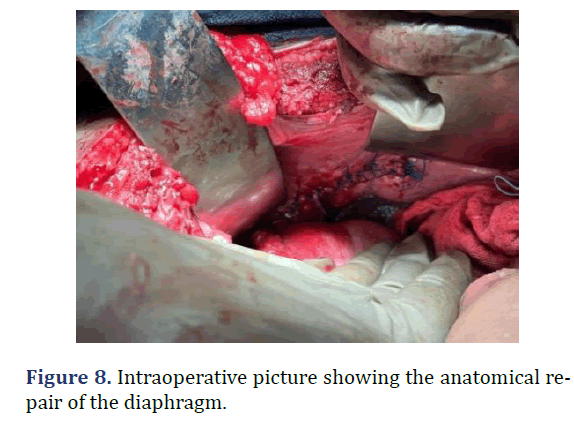
A 37 year old male admitted from emergency with dyspnea following trauma in the last day. Patient history revealed sudden onset left sided chest pain and left sided abdominal pain following trauma. Physical examination revealed tenderness in the left hypochondriac regions. Reduced breath sounds and gurgling sounds (Bowel sounds) heard in the left lower auscultatory regions of the chest. Chest radiograph revealed gastric air shadow in the left lower zone of the chest with reduced left sided lung shadow. Emergency laparotomy was done. Using rooftop incision, defect of about 10 cm × 3 cm noted in the left hemi-diaphragm. Stomach as the content of hernia noted. Reduction of the abdominal contents into the abdominal cavity with primary repair of the defect. Post-operative period was uneventful (Figures 6-8).
Case 4
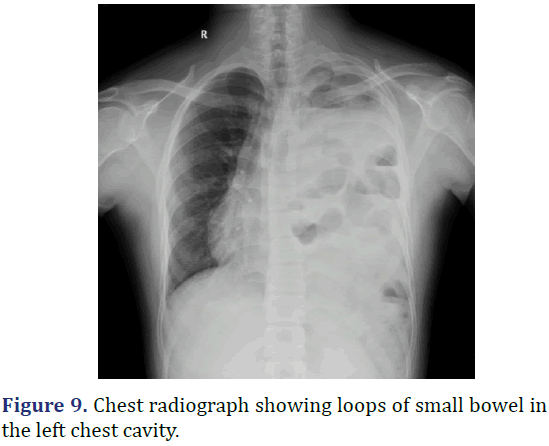
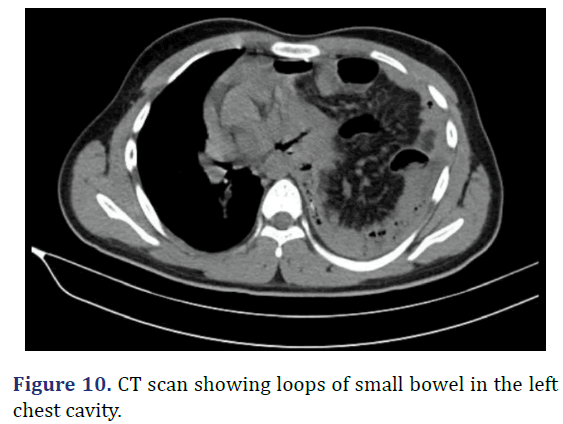
A 17 year old male was admitted with left sided chest pain, breathlessness and abdominal pain in the last 2 days. Patient history revealed left sided insidious onset chest pain, gradually progressed to severe intensity, aggravated with deep breaths. Physical examination revealed tenderness in the left hypochondrium with reduced chest movement in the left side and reduced breaths sounds in the left side. Gurgling sounds heard in the left auscultatory areas of the chest suggestive of bowel sounds. Chest radiograph showed near complete opacification of the left hemithorax with multiple bowel loops seen in the left thoracic cavity, mediastinal shift to right and left lung is compressed by the herniated abdominal contents. CT scan of the chest revealed defect in the postero-medial wall of left hemidiaphragm with herniation of the abdominal contents into the left hemithorax. Emergency exploratory laparotomy with left subcoastal with upper midline incision done. Multi-disciplinary approach to surgery with general surgery and cardiothoracic surgery teams. Appendix and ileocaecal junction noted in the left hypochondrium entire small bowel and transverse colon in the left pleural cavity. Diaphragmatic defect of 10 cm × 6 cm noted. Contents of the hernia reduced to abdominal cavity and primary repair done. Post operatively patient was in ICU for 2 days. Serial chest radiograph taken to monitor the lung expansion. Patient discharged on 21st post-operative day (Figures 9 and 10).
Case 5
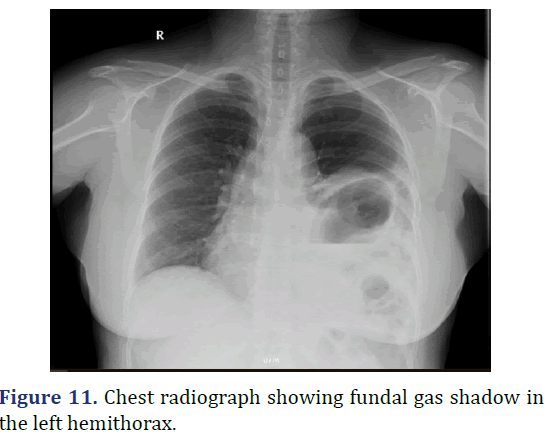
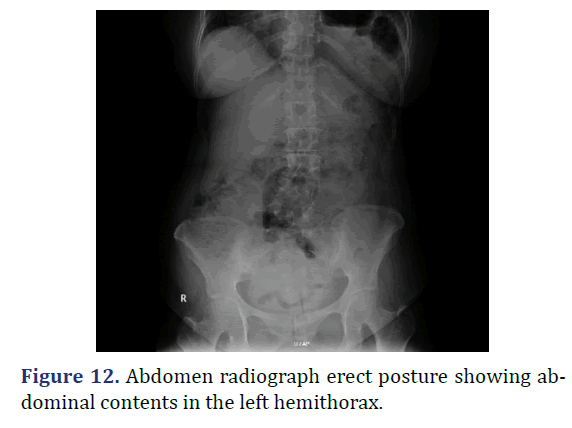
A 18 year old female admitted with abdominal pain since 5 years, breathlessness and hiccups since 3 months. Patient history revealed insidious onset abdominal pain, dull aching type gradually progressed, associated with breathlessness and hiccups. Physical examination revealed decreased chest movements on the left side, reduced breath sounds on the left side, with gurgling sounds in the left lower auscultatory areas suggestive for bowel sounds. Chest radiograph showed the reduced lung field in the left hemithorax with stomach fundus gas shadow in the thorax. Patient diagnosed to have Bochdaleck hernia. Patient underwent elective diaphragmatic hernia repair. Defect noted in the left hemidiaphragm on the posterolateral aspect with transverse colon, spleen, fundus of the stomach, omentum and part of small bowel as content of hernia. Reduction of the hernia contents, primary repair of the diaphragmatic defect with mesh reinforcement done. Postoperatively patient was in ventilatory support for 3 days, in ICU for 6 days. Serial chest radiograph taken to monitor lung expansion. Patient was discharged. Patient discharged on 21st postoperative day (Figures 11 and 12).
Results and Discussion
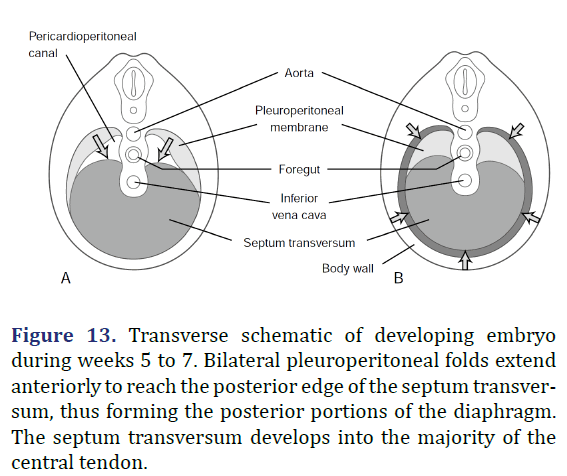
During embryogenesis, the diaphragm develops from the septum transversum and pleuroperitoneal folds [7]. The septum transversum is a single ventral membrane which separates the pericardium from the rest of the thorax and develops as the central trileaflet of the diaphragmatic tendon [8]. One of the trileaflet tendons is situated within the right hemithorax, one within the left hemithorax, and the third beneath the pericardium. The dorsolateral portions of the diaphragm start to develop with the formation of pleuroperitoneal folds. The mesothelium of the pleura binds to the mesothelium of the peritoneum forming a membrane only two cell layers deep. In the 7th week of life, myotomes from C3, C4, and C5 migrate from the lateral border toward the center of each hemithorax within the interspace between these two mesothelial layers. The outer rim of muscle of the diaphragm is from myotomes that carry nerve innervation from T7 through T12 [8]. The intestines return to the abdomen from the yolk sac in the 10th week of life and will be displaced into the chest if the diaphragm has not successfully formed (Figure 13) [8].
Figure 13. Transverse schematic of developing embryo during weeks 5 to 7. Bilateral pleuroperitoneal folds extend anteriorly to reach the posterior edge of the septum transversum, thus forming the posterior portions of the diaphragm. The septum transversum develops into the majority of the central tendon.
The pathogenesis of CDH is also unknown. Normally, before the return of the bowel from the umbilical cord to the abdominal cavity, which occurs during the 10th week of gestation, it is necessary for the pleuroperitoneal canal and the lumbocostal triangle to firmly close, which happens between the 8th and 10th weeks. If such closure does not take place, the bowel will pass through the pleuroperitoneal canal, sometimes also through the lumbocostal triangle, and will invade the chest, resulting in a “herniation” without a hernia sac. If there is only a membranous closure, the same phenomenon will happen, albeit with a hernia sacs that may, or may not, rupture sometime later [6].
The prevailing theory as to CDH development has been that the incomplete closure of the pleuroperitoneal canal was a primarily diaphragmatic defect and that the abdominal viscera herniated to the thorax impeded normal lung development and resulted in the pulmonary hypoplasia and hypertension observed almost universally in association with CDH [6].
Nonetheless, the foremost notion today is that the primary defect is not in the diaphragm but in the lung buds, and that the diaphragmatic defect would actually be secondary to a primary pulmonary hypoplasia [6].
Caval opening transmits the inferior vena cava and the half of right Phrenic nerve. Oesophageal opening transmits oesophagus, right and left vagi and the oesphageal branches of left gastric artery and accompanying veins. Aortic opening transmits vena azygos, the thoracic duct and the aorta (Table 1) [9].
| Embryonic structure | Time (wk) | Portion formed | Diaphragmatic defect |
|---|---|---|---|
| Transverse septum | 3 and 4 | Central tendon | Pericardial hernia (ventral defect) |
| Pleuroperitoneal membranes | 7 and 8 | Primitive diaphragm | Bochdalek hernia (posterolateral defect) |
| Mediastinum (dorsal mesentery) | — | Median portion and crura | — |
| Body wall | 9 to 12 | Muscle periphery (ventral-lateral and dorsal) | Morgagni hernia (parasternal defect) and eventration |
Ninety percent of the patients with CDH are symptomatic within the first 24 hours of life. However, this disease may first manifest at any age and, more rarely, go unnoticed until very late in life or even never be diagnosed [10,11]. When a child is symptomatic within the first 24 hours of life, the main clinical manifestation is respiratory distress. The earlier the onset of signs and symptoms, the more severe the pulmonary disease. Tachypnea associated with sternal, subcostal, and supraclavicular retraction is common. Cyanosis and pallor are also frequent. Apgar scores tend to be low. The abdomen is often scaphoid because of the migration of abdominal viscera to the chest. However, because of possible bowel distention inside the abdominal cavity, the abdomen may assume a normal appearance with time. The chest may be asymmetrical, larger on the side of the hernia, especially after the bowel fills with gas. The heart sounds are commonly dislocated to the contralateral side of the hernia. Sometimes the same happens with the trachea. In the ipsilateral hemithorax, the respiratory sounds may be diminished or absent altogether, and bowel sounds may be present. There may be hemodynamic instability, with a tendency to arterial hypotension, because of a decreased venous return to the heart resulting from the mediastinal deviation or because of right heart failure due to pulmonary hypertension. Occasionally, mediastinal deviation can also lead to superior vena cava syndrome[12]. There may be manifestations stemming from perforations or strangulation, or both, of a hollow viscus, gastric, or midgut volvulus, or rupture of a herniated spleen, such as Gastro Intestinal (GI) obstructions, empyema, hemothorax, fever, arterial hypotension, coagulopathy, anemia, or hypovolemic shock [6].
The spectrum of manifestations of late-presentation CDH is quite broad, including, in addition to GI obstruction and respiratory distress, sudden death, growth retardation, perforations or strangulations of intrathoracic hollow viscera (which may lead to sepsis, empyema, pneumothorax, and hemothorax), rupture of a herniated spleen (with hemothorax, anemia, and possibly hypovolemic shock), airway infections or recurrent pneumonias, urinary tract obstruction due to herniation of the ureter, chest pain, abdominal pain, vomiting, diarrhea, anorexia, acute abdomen, intrathoracic appendicitis, and other rare presentations [6,10,11].
Surgery typically involves patch repair or primary closure of the diaphragmatic defect via an abdominal approach. If adhesions between the herniated content and viscera of the chest are suspected, thoracotomy or combined thoracoabdominal approach can be taken [6].
Foramen of Morgagni hernias are frequently repaired from an abdominal approach. Both laparoscopic and thoracoscopic repairs have been described [12-14]. A small rim of the internal transthoracic muscle is frequently found behind the sternum and is sutured to the diaphragm to close the hernia. We perform this closure with interrupted mattress sutures using a heavy nonabsorbable suture. Alternatively, the posterior rectus sheath or a rib can be used to help close the hernia. Rarely, the hernia cannot be closed without tension, and a patch is used. Foramen of Bochdalek hernias are mostly small and repaired primarily. This can be done minimally invasively. Larger defects may require patch [6].
Repair of congenital diaphragmatic hernia involving the central trileaflet is straight forward, because of the laxity of the central tendon. Primary repair is frequently possible. Very large defect requires patching [6].
The initial step in operative repair of a hiatal hernia is to clearly delineate the muscular limits of the hiatal defect. Laparoscopic approaches have proved somewhat superior in this goal of identifying the limits of hiatal hernia [6].
The fat between the proximal stomach and the diaphragmatic muscle needs to be removed. Dividing the muscular fibers of the left diaphragmatic crus in a straight lateral manner can enlarge tight hiatal rings. This will not divide the branches of the phrenic nerve that lie far anteriorly, nor the phrenic vein, which lies medially. The left inferior phrenic artery may be divided without causing ischemia because anastomosing vessels continue to perfuse the area. The enlargement of the tight hiatal ring can greatly facilitate the reduction of a large bulky hernia. Alternatively, a red rubber catheter can be passed across the neck of the hiatal hernia and air insufflated within the sac to facilitate reduction of the hernia contents. This breaks the natural vacuum that occurs in trying to reduce the abdominal contents through the hiatal neck [6].
The diaphragmatic defect needs to be closed as part of the surgical repair of the hiatal hernia. Closure of the dilated esophageal diaphragmatic hiatus is frequently performed by placement of sutures reapproximating the right and left crura of the diaphragm. In large hiatal hernias, this defect can be sizable. If the fibers of the diaphragmatic crura have been thinned, we frequently use pledgeted nonabsorbable sutures to reapproximate the right and left muscle bundles. The tenant of all hernia repairs is to prevent tension at closure, and some defects may require patch or mesh reinforcement [6].
Conclusion
Congenitial Diaphragmatic Hernia in adults is relatively rare. Clinical signs such as bowel sounds in the respiratory auscultatory areas should raise a high index of suspicion of diaphragmatic hernia. Trauma to the abdomen can lead to rupture of the diaphragm and hernia of the abdominal contents. A chest radiograph can lead to the diagnosis of diaphragmatic hernia. CT thorax gives information about the content of hernia and any adhesion between the abdominal viscera and thorax. When a diaphragmatic hernia in adults is diagnosed, surgery is the treatment of choice, above all in emergency setting. Associated pulmonary hypoplasia and pulmonary hypertension can complicate recovery even if surgery is done well. Reduction of the hernial content into the abdomen and primary repair of the diaphragmatic defect with mesh reinforcement is our choice. A multidisciplinary approach is advised when adhesions between abdominal viscera and thorax are suspected.
Limitation
Only five patients were admitted with diagnosis of diaphragmatic hernia.
References
- Perrone G, Giuffrida M, Annicchiarico A, Bonati E, Del Rio P, Testini M, et al. Complicated diaphragmatic hernia in emergency surgery: Systematic review of the literature. World J Surg 2020; 44(12):4012-31.
[Crossref] [Google Scholar] [Pubmed]
- Townsend CM, Beauchamp RD, Evers BM, Mattox KL. Sabiston textbook of surgery. Elsevier Health Sciences, 2016.
- Fischer JE. Fischer’s Mastery of Surgery. Wolters Kluwer, 2019.
- Cordier AG, Russo FM, Deprest J, Benachi A. Prenatal diagnosis, imaging, and prognosis in Congenital Diaphragmatic Hernia. Semin perinatol 2020; 44(1):51163.
[Crossref] [Google Scholar] [Pubmed]
- Irish MS, Holm BA, Glick PL. Congenital Diaphragmatic Hernia: A Historical Review. Clin Perinatol 1996; 23(4):625-53.
[Crossref] [Google Scholar] [Pubmed]
- Sellke FW, Del Nido PJ, Swanson SJ. Sabiston and spencer surgery of the chest. Saunders, Elsevier, 2010; 473-515.
- Schumpelick V, Steinau G, Schlüper I, Prescher A. Surgical embryology and anatomy of the diaphragm with surgical applications. Surg Clin North Am 2000; 80(1):213-39.
[Crossref] [Google Scholar] [Pubmed]
- Larsen W. Human embryology. 2nd edn. New York: Churchill Livingstone, 1997; 136-137.
- Decker GA. The groin and scrotum. Lee Mc Gregor’s Synopsis of Surgical Anatomy. Twelfth. John Wright and Sons Ltd., 1999; 152-161.
- Osebold WR, Soper RT. Congenital posterolateral diaphragmatic hernia past infancy. Am J Surg 1976; 131(6):748-54.
[Crossref] [Google Scholar] [Pubmed]
- Fan LL, Graham LM. Radiological cases of the month. Arch Pediatr Adolesc Med 1994; 148(2):205-6.
- Giacoia GP. Right-sided diaphragmatic hernia associated with superior vena cava syndrome. Am J Perinatol 1994; 11(02):129-31.
- Huntington TR. Laparoscopic transabdominal preperitoneal repair of a hernia of Morgagni. J Laparoendosc Surg 1996; 6(2):131-3.
[Crossref] [Google Scholar] [Pubmed]
- Hussong RL, Landreneau RJ, Cole FH. Diagnosis and repair of a Morgagni hernia with video assisted thoracic surgery. Ann Thorac Surg 1997; 63(5):1474-5.
[Crossref] [Google Scholar] [Pubmed]
Copyright: © 2023 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.